Acute Crit Care.
2022 Nov;37(4):550-560. 10.4266/acc.2022.00682.
The frequency and seasonal distribution of viral infection in patients with community-acquired pneumonia and its impact on the prognosis
- Affiliations
-
- 1Army Training Center, Republic of Korea Army, Nonsan, Korea
- 2Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Dankook University Hospital, Dankook University College of Medicine, Cheonan, Korea
- KMID: 2540143
- DOI: http://doi.org/10.4266/acc.2022.00682
Abstract
- Background
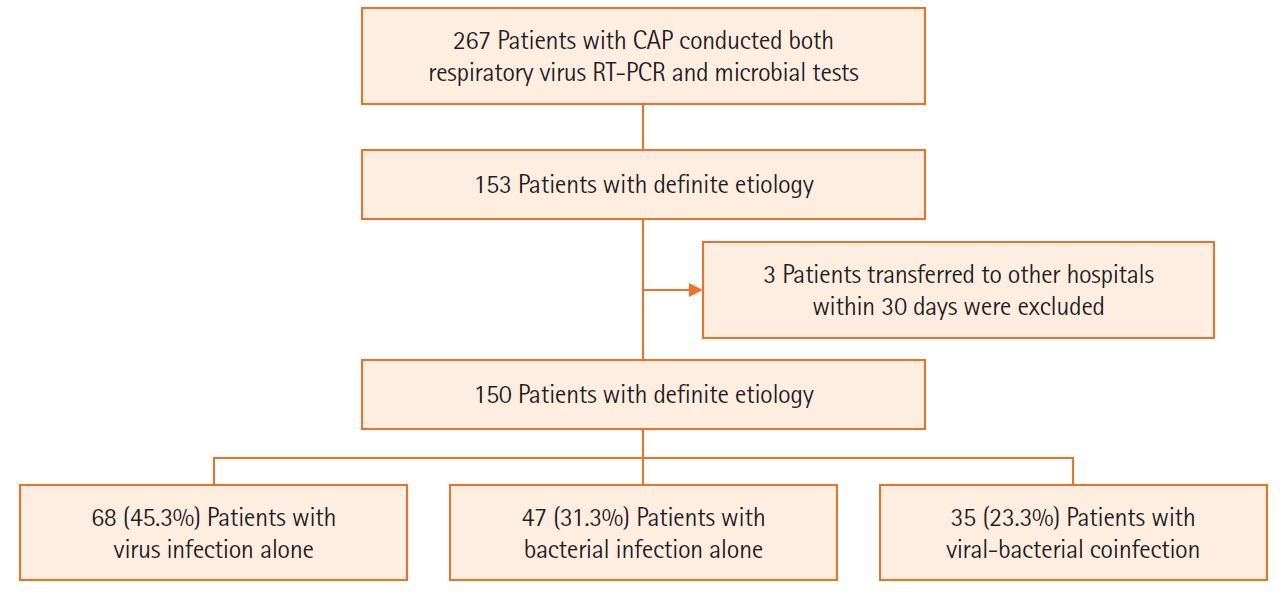
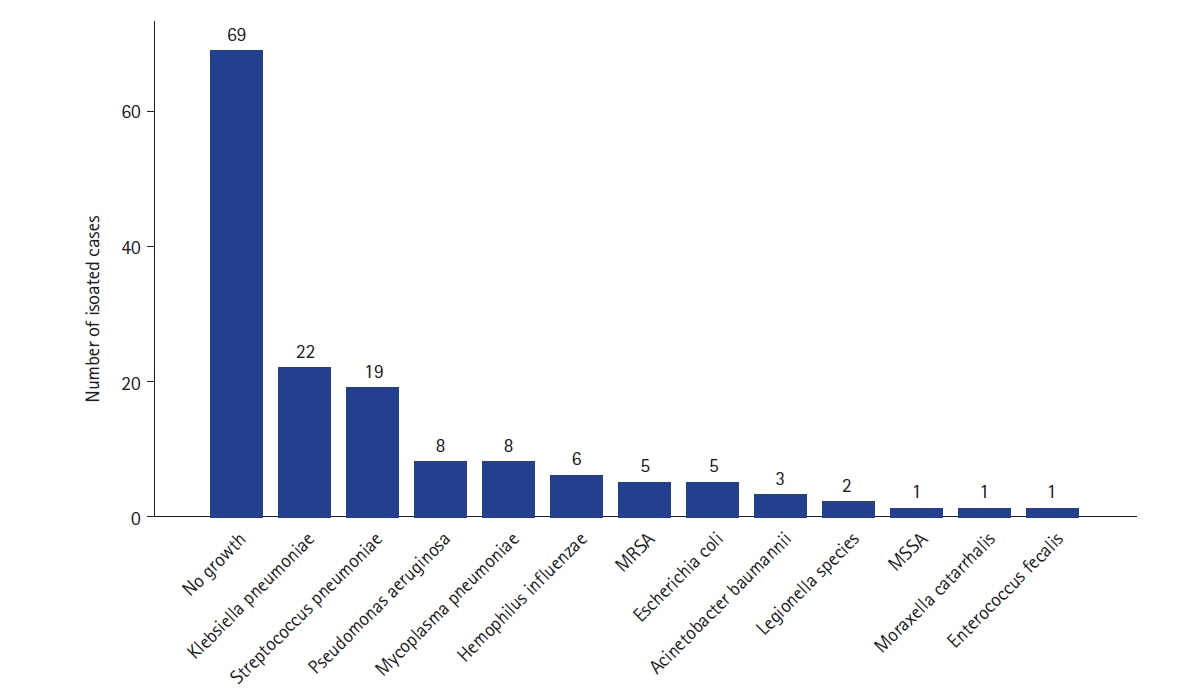
Studies on the effects of viral coinfection on bacterial pneumonia are still scarce in South Korea. This study investigates the frequency and seasonal distribution of virus infection and its impact on the prognosis in patients with community-acquired pneumonia (CAP). Methods: The medical records of CAP patients with definite etiology, such as viruses and bacteria, were retrospectively reviewed. Their epidemiologic and clinical characteristics, microbiologic test results, the severity of illness, and 30-day mortality were analyzed. Results: Among 150 study subjects, 68 patients (45.3%) had viral infection alone, 47 (31.3%) had bacterial infection alone, and 35 (23.3%) had viral-bacterial coinfection, respectively. Among 103 patients with viral infections, Influenza A virus (44%) was the most common virus, followed by rhinovirus (19%), influenza B (13%), and adenovirus (6%). The confusion-urea-respiratory rateblood pressure-age of 65 (CURB-65) score of the viral-bacterial coinfection was higher than that of the viral infection (median [interquartile range]: 2.0 [1.0–4.0] vs. 2.0 [0.3–3.0], P=0.029). The 30-day mortality of the viral infection alone group (2.9%) was significantly lower than that of bacterial infection alone (19.1%) and viral-bacterial coinfection (25.7%) groups (Bonferroni-corrected P<0.05). Viral-bacterial coinfection was the stronger predictor of 30-day mortality in CAP (odds ratio [OR], 18.9; 95% confidence interval [CI], 3.0–118.3; P=0.002) than bacterial infection alone (OR, 6.3; 95% CI, 1.1–36.4; P=0.041), compared to viral infection alone on the multivariate analysis. Conclusions: The etiology of viral infection in CAP is different according to regional characteristics. Viral-bacterial coinfection showed a worse prognosis than bacterial infection alone in patients with CAP.
Figure
Reference
-
1. Jameson JL. Harrison’s principles of internal medicine. 20th ed. New York: McGraw-Hill;2018.2. Yoon HK. Changes in the epidemiology and burden of community-acquired pneumonia in Korea. Korean J Intern Med. 2014; 29:735–7.
Article3. Vital Statistics Division; Statistics Korea, Shin HY, Kim J, Lee S, Park MS, et al. Cause-of-death statistics in 2018 in the Republic of Korea. J Korean Med Assoc. 2020; 63:286–97.
Article4. Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect. 2013; 67:11–8.
Article5. Wunderink RG, Waterer GW. Clinical practice: community-acquired pneumonia. N Engl J Med. 2014; 370:543–51.6. Seki M, Kosai K, Yanagihara K, Higashiyama Y, Kurihara S, Izumikawa K, et al. Disease severity in patients with simultaneous influenza and bacterial pneumonia. Intern Med. 2007; 46:953–8.
Article7. Kang YS, Ryoo SR, Byun SJ, Jeong YJ, Oh JY, Yoon YS. Antimicrobial resistance and clinical outcomes in nursing home-acquired pneumonia, compared to community-acquired pneumonia. Yonsei Med J. 2017; 58:180–6.
Article8. Choi SH, Hong SB, Ko GB, Lee Y, Park HJ, Park SY, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012; 186:325–32.
Article9. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015; 373:415–27.
Article10. Chong YP, Jung KS, Lee KH, Kim MN, Moon SM, Park S, et al. The bacterial etiology of community-acquired pneumonia in Korea: a nationwide prospective multicenter study. Infect Chemother. 2010; 42:397–403.
Article11. Yoo KH, Yoo CG, Kim SK, Jung JY, Lee MG, Uh ST, et al. Economic burden and epidemiology of pneumonia in Korean adults aged over 50 years. J Korean Med Sci. 2013; 28:888–95.
Article12. Kim JE, Kim UJ, Kim HK, Cho SK, An JH, Kang SJ, et al. Predictors of viral pneumonia in patients with community-acquired pneumonia. PLoS One. 2014; 9:e114710.
Article13. Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009; 200:546–54.
Article14. Kovacs EJ, Boe DM, Boule LA, Curtis BJ. Inflammaging and the lung. Clin Geriatr Med. 2017; 33:459–71.
Article15. Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019; 4:e123158.
Article16. Ongradi J, Kovesdi V. Factors that may impact on immunosenescence: an appraisal. Immun Ageing. 2010; 7:7.
Article17. Ron-Harel N, Notarangelo G, Ghergurovich JM, Paulo JA, Sage PT, Santos D, et al. Defective respiration and one-carbon metabolism contribute to impaired naive T cell activation in aged mice. Proc Natl Acad Sci U S A. 2018; 115:13347–52.18. Salam N, Rane S, Das R, Faulkner M, Gund R, Kandpal U, et al. T cell ageing: effects of age on development, survival & function. Indian J Med Res. 2013; 138:595–608.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The clinical and microbial characteristics of healthcare-associated pneumonia
- Community-acquired pneumonia in elderly patients
- Disease Burden and Etiologic Distribution of Community-Acquired Pneumonia in Adults: Evolving Epidemiology in the Era of Pneumococcal Conjugate Vaccines
- Severe Adenovirus Pneumonia
- Respiratory Review of 2010: Pneumonia