Clin Exp Otorhinolaryngol.
2023 Feb;16(1):37-48. 10.21053/ceo.2022.01172.
Development of a Serum-Free Culture Method for Endothelial Cells of the Stria Vascularis and Their Pro-Inflammatory Secretome Changes Induced by Oxidative Stress
- Affiliations
-
- 1Otorhinolaryngology Hospital, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- KMID: 2539767
- DOI: http://doi.org/10.21053/ceo.2022.01172
Abstract
Objectives
. Reactive oxygen species in the stria vascularis (SV) of the cochlea may be involved in the pathogenesis of sensorineural hearing loss. However, the effects of oxidative stress on SV endothelial cells (SV-ECs) remain largely unknown, and no feasible in vitro cell culture model exists for the functional study of SV-ECs.
Methods
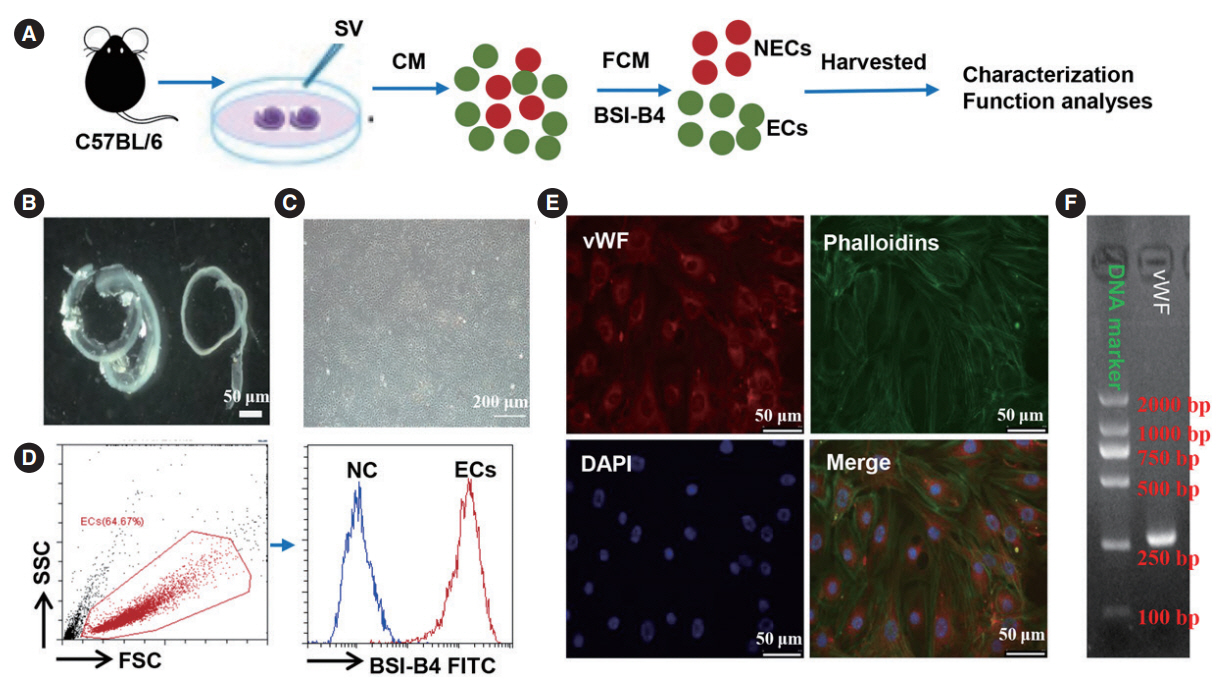
. We isolated primary SV-ECs from the SV of neonatal mice. The apoptosis-reducing effects of fibronectin in SV-ECs cultured with serum-free medium were determined using β-galactosidase staining and flow cytometry. SV-ECs incubated in serum-free medium were treated with various H2O2 concentrations to evaluate the effects of H2O2 on their viability. The secretome of SV-ECs treated with or without H2O2 (100 μM or 500 μM) was analyzed using high-resolution mass spectrometry. The function of the SV-EC secretome was evaluated by a macrophage assay.
Results
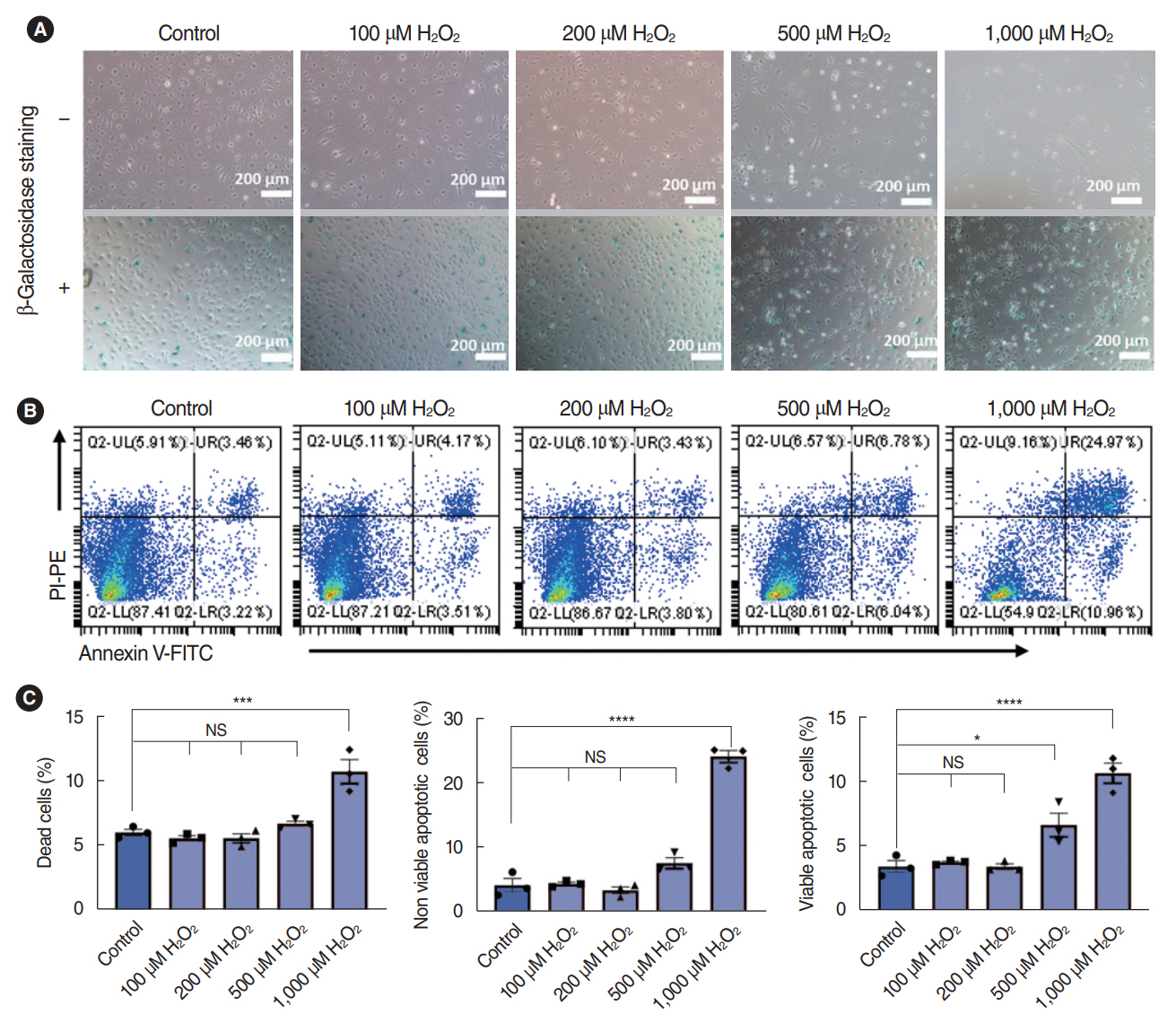
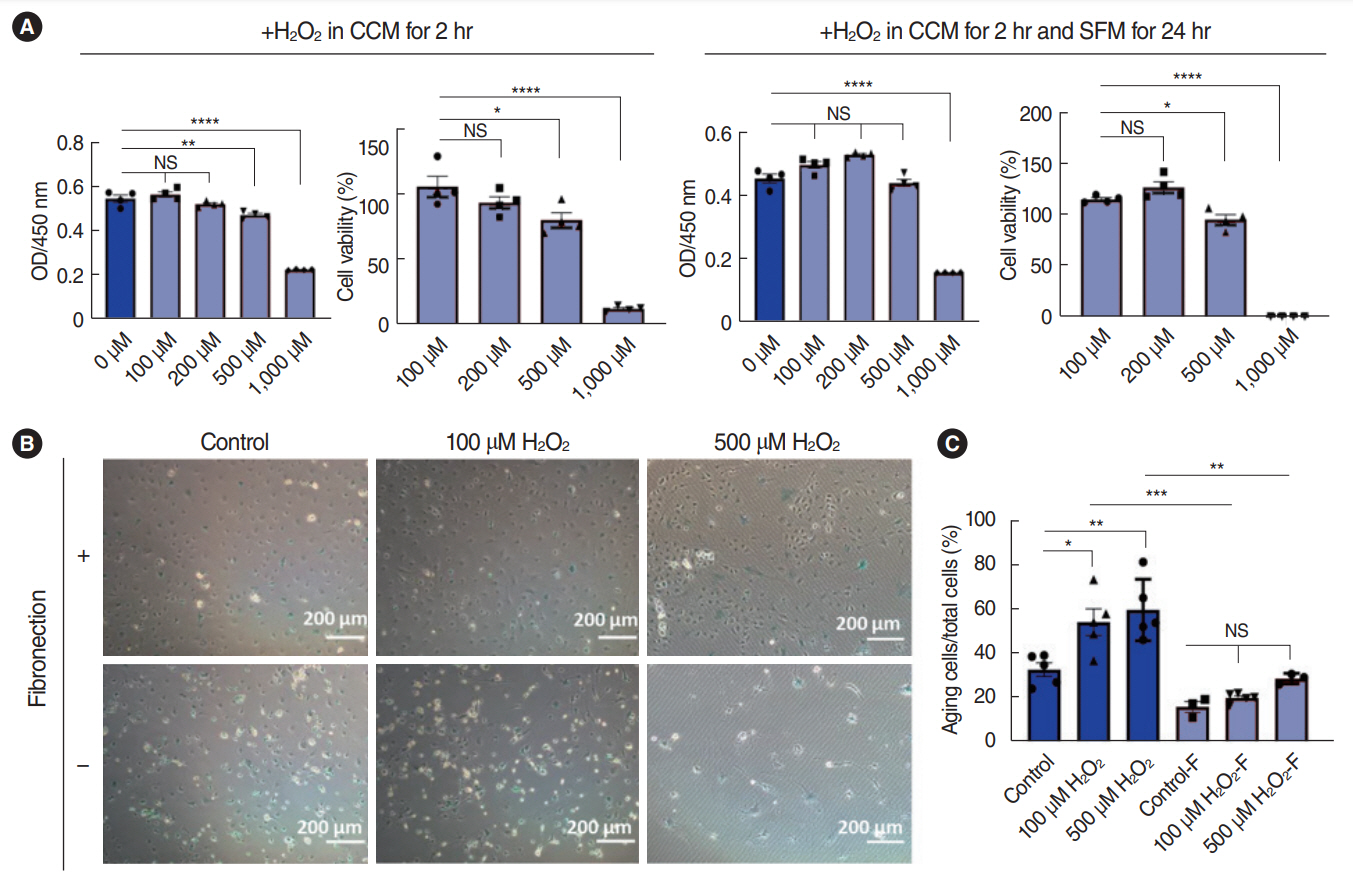
. We successfully isolated and characterized the SV-ECs. Treatment with H2O2 at concentrations up to 500 μM for 2 hours and further incubation with serum-free medium in plates precoated with fibronectin showed no significant effect on apoptosis. Compared to the control SV-ECs, the amount of differential proteins in the secretome of SV-ECs stimulated with 500 μM H2O2 was much higher than in those treated with 100 μM H2O2. Kyoto Encyclopedia of Genes and Genomes and Gene Ontology analyses suggested that the proteins differentially expressed in SV-ECs treated with 500 μM H2O2 were involved in the regulation of multiple signaling pathways and cellular processes. The secretome of H2O2-stimulated SV-ECs exhibited significant pro-inflammatory effects on macrophages.
Conclusion
. We successfully established an in vitro serum-free culture method, identified the differential proteins released by oxidative stress-induced ECs and their functions, and revealed the pro-inflammatory effects of the secretome of H2O2-stimulated SV-ECs. Therefore, SV-ECs might elicit immunoregulatory effects on bystander cells in the microenvironment of oxidative stress-induced cochlea, especially cochlear macrophages.
Keyword
Figure
Cited by 1 articles
-
Reprogramming Macrophage Phenotypes With Photobiomodulation for Improved Inflammation Control in ENT Organ Tissues
Ken Woo, Yeon Soo Kim, Celine Abueva, Seung Hoon Woo
Clin Exp Otorhinolaryngol. 2025;18(1):1-13. doi: 10.21053/ceo.2024.00286.
Reference
-
1. Tordrup D, Smith R, Kamenov K, Bertram MY, Green N, Chadha S, et al. Global return on investment and cost-effectiveness of WHO’s HEAR interventions for hearing loss: a modelling study. Lancet Glob Health. 2022; Jan. 10(1):e52–62.
Article2. Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res. 2016; Aug. 338:52–63.
Article3. Neng L, Zhang F, Kachelmeier A, Shi X. Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J Assoc Res Otolaryngol. 2013; Apr. 14(2):175–85.
Article4. Yu W, Zong S, Du P, Zhou P, Li H, Wang E, et al. Role of the stria vascularis in the pathogenesis of sensorineural hearing loss: a narrative review. Front Neurosci. 2021; Nov. 15:774585.
Article5. Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017; Jan. 120(1):179–206.
Article6. Zhou B, Magana L, Hong Z, Huang LS, Chakraborty S, Tsukasaki Y, et al. The angiocrine Rspondin3 instructs interstitial macrophage transition via metabolic-epigenetic reprogramming and resolves inflammatory injury. Nat Immunol. 2020; Nov. 21(11):1430–43.
Article7. Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006; Apr. 92(4):441–4.
Article8. Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2010; Oct. 411(19-20):1412–20.9. Wettschureck N, Strilic B, Offermanns S. Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev. 2019; Jul. 99(3):1467–525.
Article10. de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012; Apr. 1:18396.11. Neng L, Zhang W, Hassan A, Zemla M, Kachelmeier A, Fridberger A, et al. Isolation and culture of endothelial cells, pericytes and perivascular resident macrophage-like melanocytes from the young mouse ear. Nat Protoc. 2013; Apr. 8(4):709–20.
Article12. Fujimoto C, Yamasoba T. Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxid Med Cell Longev. 2014; 2014:582849.
Article13. Fetoni AR, Paciello F, Rolesi R, Paludetti G, Troiani D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: oxidative stress and ROS signaling. Free Radic Biol Med. 2019; May. 135:46–59.14. Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal. 2011; Feb. 14(3):459–68.15. Wong AC, Ryan AF. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci. 2015; Apr. 7:58.16. Shin SA, Lyu AR, Jeong SH, Kim TH, Park MJ, Park YH. Acoustic trauma modulates cochlear blood flow and vasoactive factors in a rodent model of noise-induced hearing loss. Int J Mol Sci. 2019; Oct. 20(21):5316.17. Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced celluar injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002; Sep. 3(3):248–68.18. Shi X, Nuttall AL. Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Res. 2003; Mar. 967(1-2):1–10.19. Xu ZZ, Li ZJ, Du LX, Li J, Wang LY. Using bovine pituitary extract to increase proliferation of keratocytes and maintain their phenotype in vitro. Int J Ophthalmol. 2013; Dec. 6(6):758–65.20. Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010; 26:397–419.
Article21. Shi X, Dai C, Nuttall AL. Altered expression of inducible nitric oxide synthase (iNOS) in the cochlea. Hear Res. 2003; Mar. 177(1-2):43–52.
Article22. Park WH. The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. Int J Mol Med. 2013; Feb. 31(2):471–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphological changes of the stria vascularis in the absence ofadrenocorticosteroid hormones
- Pro-Oxidative and Inflammatory Actions of Extracellular Hemoglobin and Heme: Molecular Events and Implications for Alzheimer’s and Parkinson Disease
- The expression of voltage-dependent K+ channels in stria vascularis of the guinea pig cochlea
- Expression and Distribution of Tumor Necrosis Factor-Alpha in Mice Cochlea Exposed to Noise
- The Changes of Na+-K+ ATPase Activity and Ultrastructure of Endolymphatic Secretory Epithelium by Local and Systemic Streptomycin Treatment