Int J Stem Cells.
2023 Feb;16(1):52-65. 10.15283/ijsc22014.
Mesenchymal Stem Cells Ameliorate Fibrosis by Enhancing Autophagy via Inhibiting Galectin-3/Akt/mTOR Pathway and by Alleviating the EMT via Inhibiting Galectin-3/Akt/GSK3β/Snail Pathway in NRK-52E Fibrosis
- Affiliations
-
- 1Department of Human Anatomy and Histoembryology, School of Basic Medical Sciences, Southwest Medical University, Luzhou, China
- 2Department of Otorhinolaryngology Head and Neck Surgery, Chongqing University Fuling Hospital, Chongqing, China
- 3Department of Chemistry, School of Basic Medical Sciences, Southwest Medical University, Luzhou, China
- KMID: 2539621
- DOI: http://doi.org/10.15283/ijsc22014
Abstract
- Background and Objectives
Epithelial-Mesenchymal transition (EMT) is one of the origins of myofibroblasts in renal interstitial fibrosis. Mesenchymal stem cells (MSCs) alleviating EMT has been proved, but the concrete mechanism is unclear. To explore the mechanism, serum-free MSCs conditioned medium (SF-MSCs-CM) was used to treat rat renal tubular epithelial cells (NRK-52E) fibrosis induced by transforming growth factor-β1 (TGF-β1) which ameliorated EMT.
Methods and Results
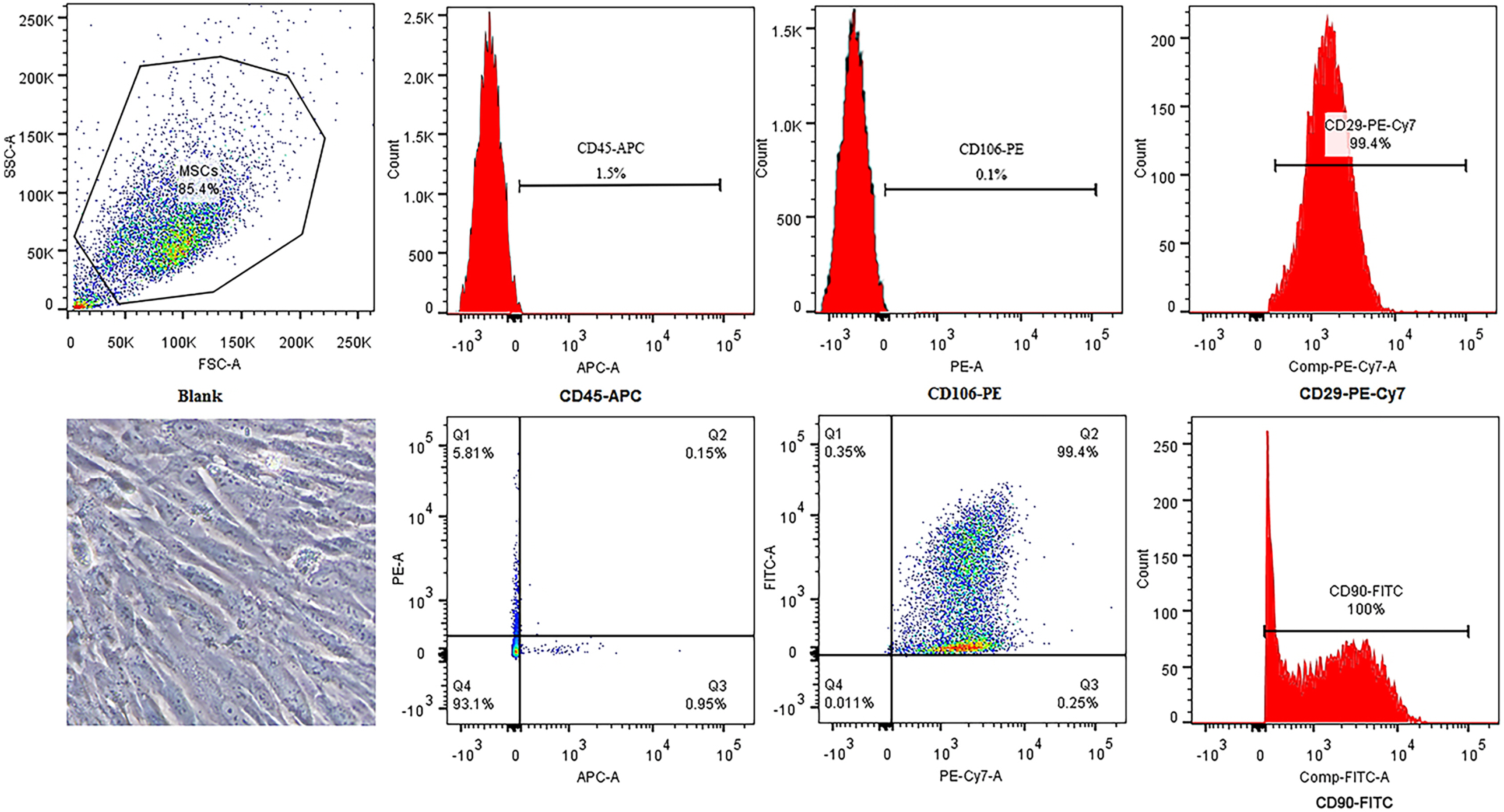
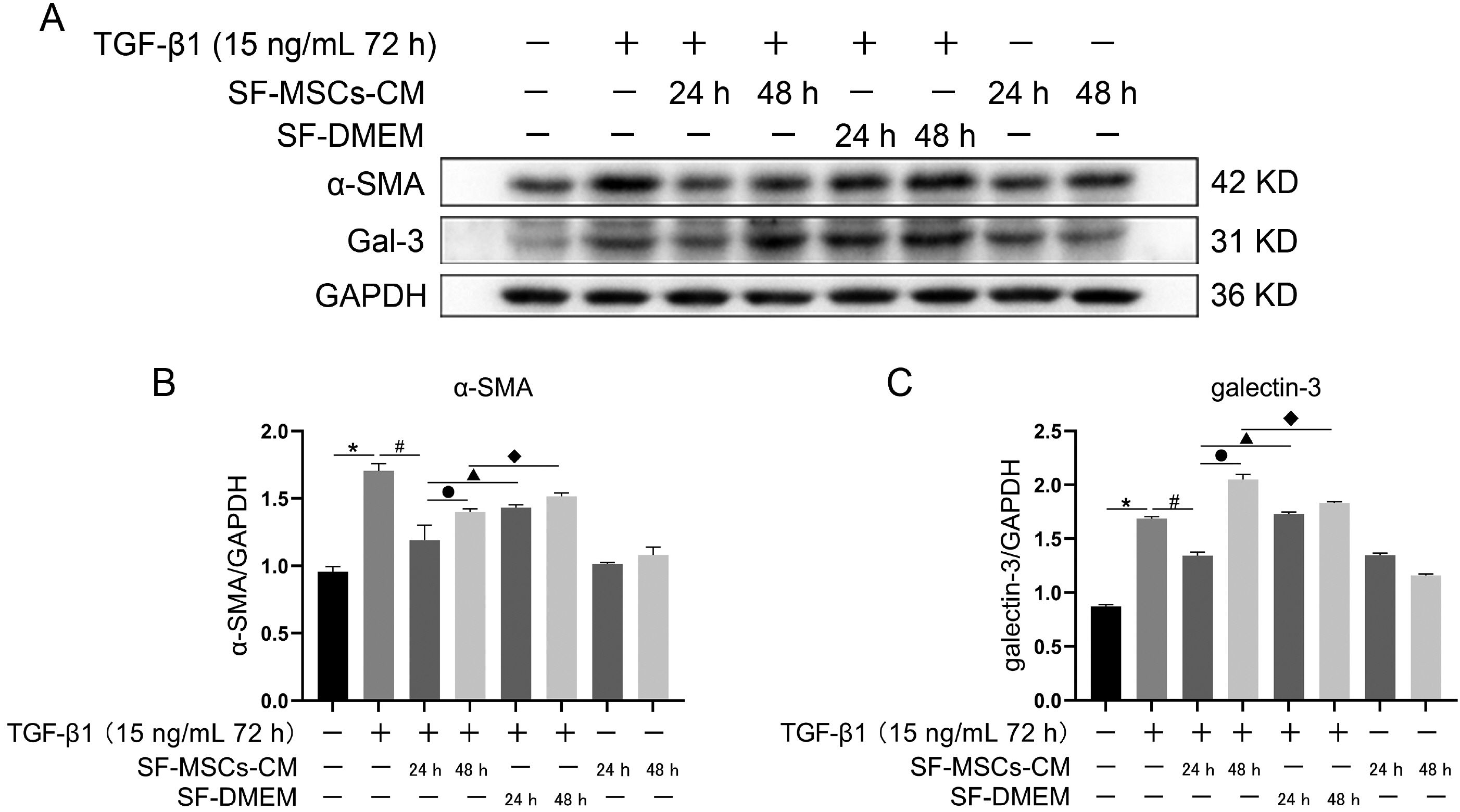
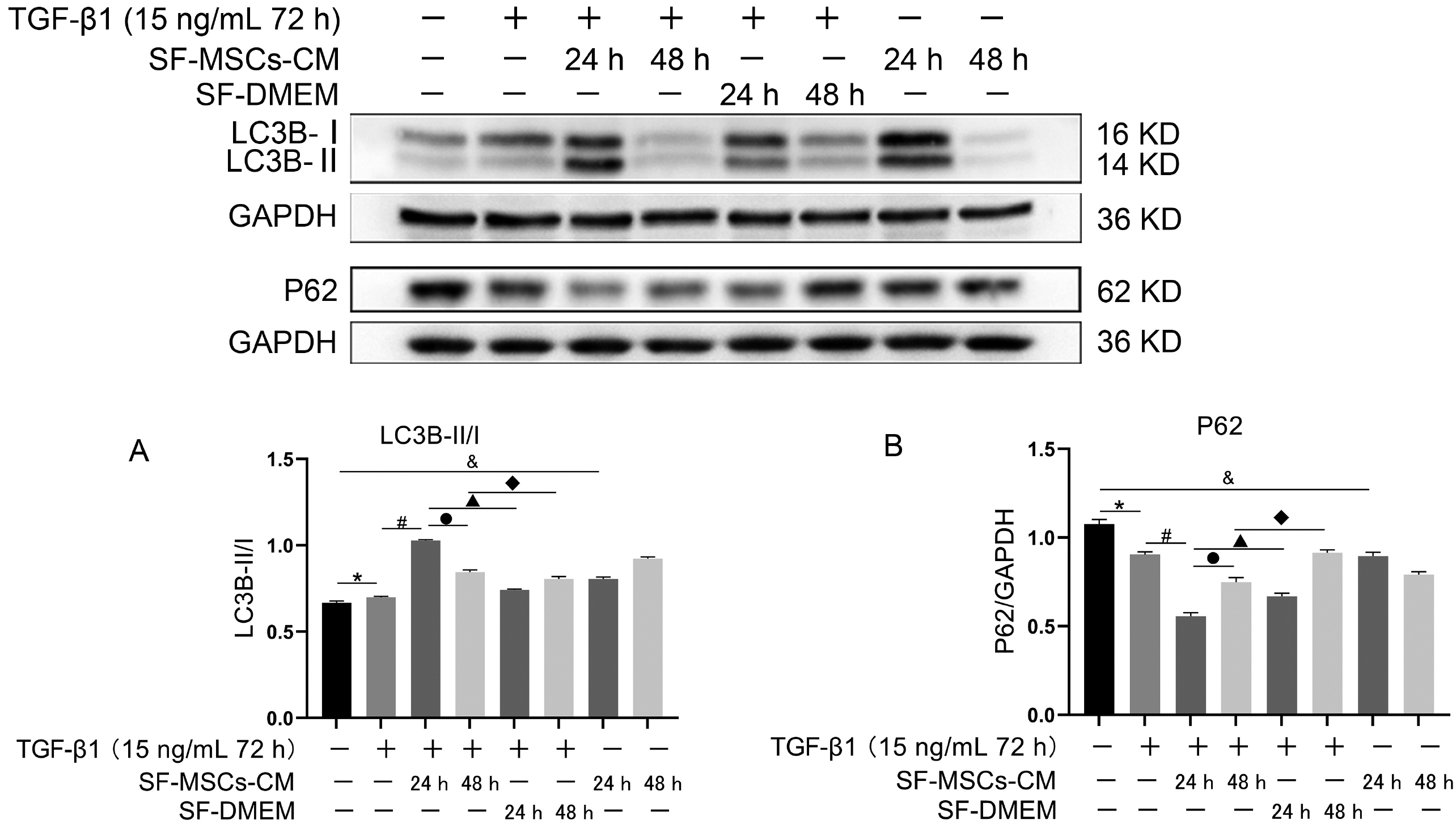
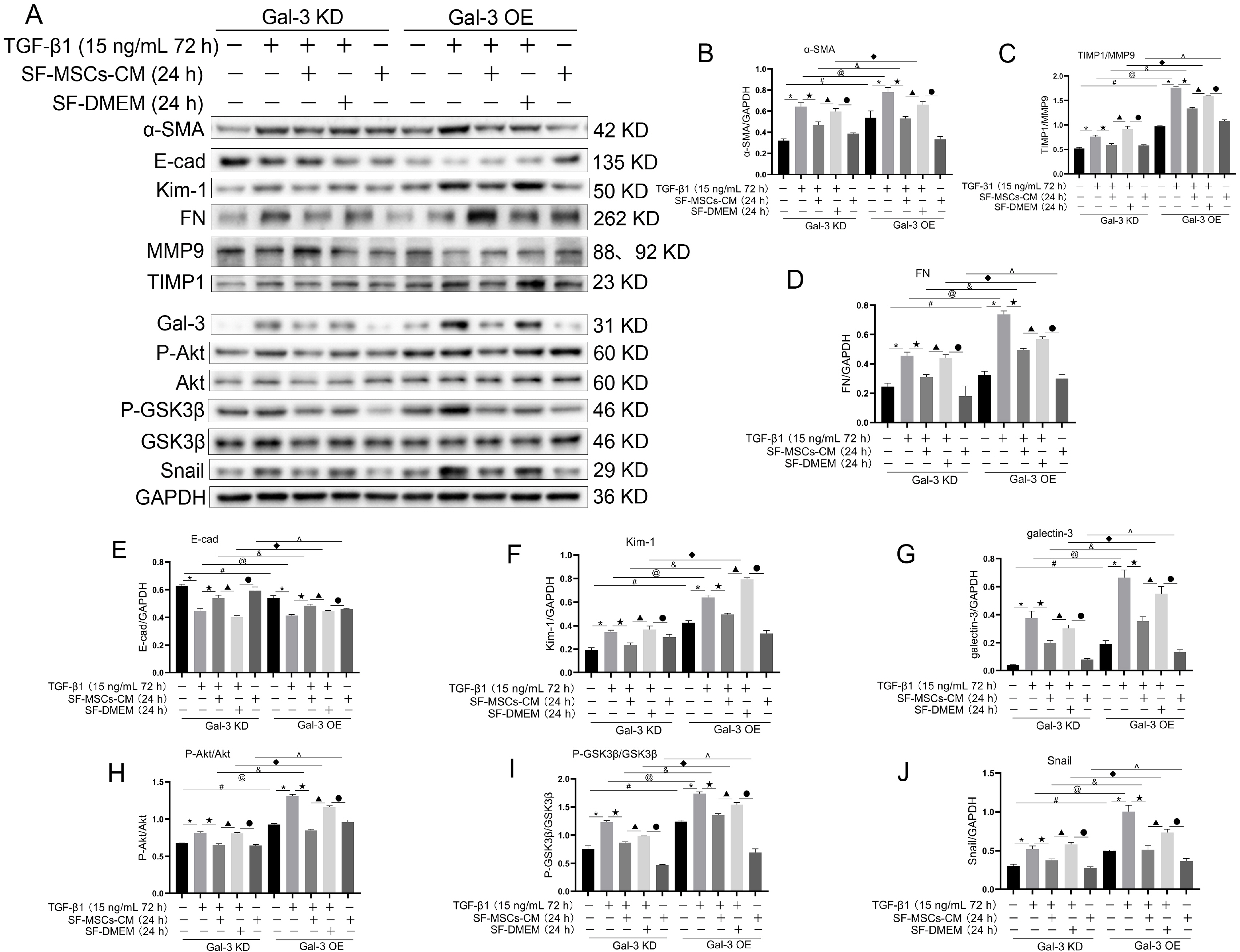
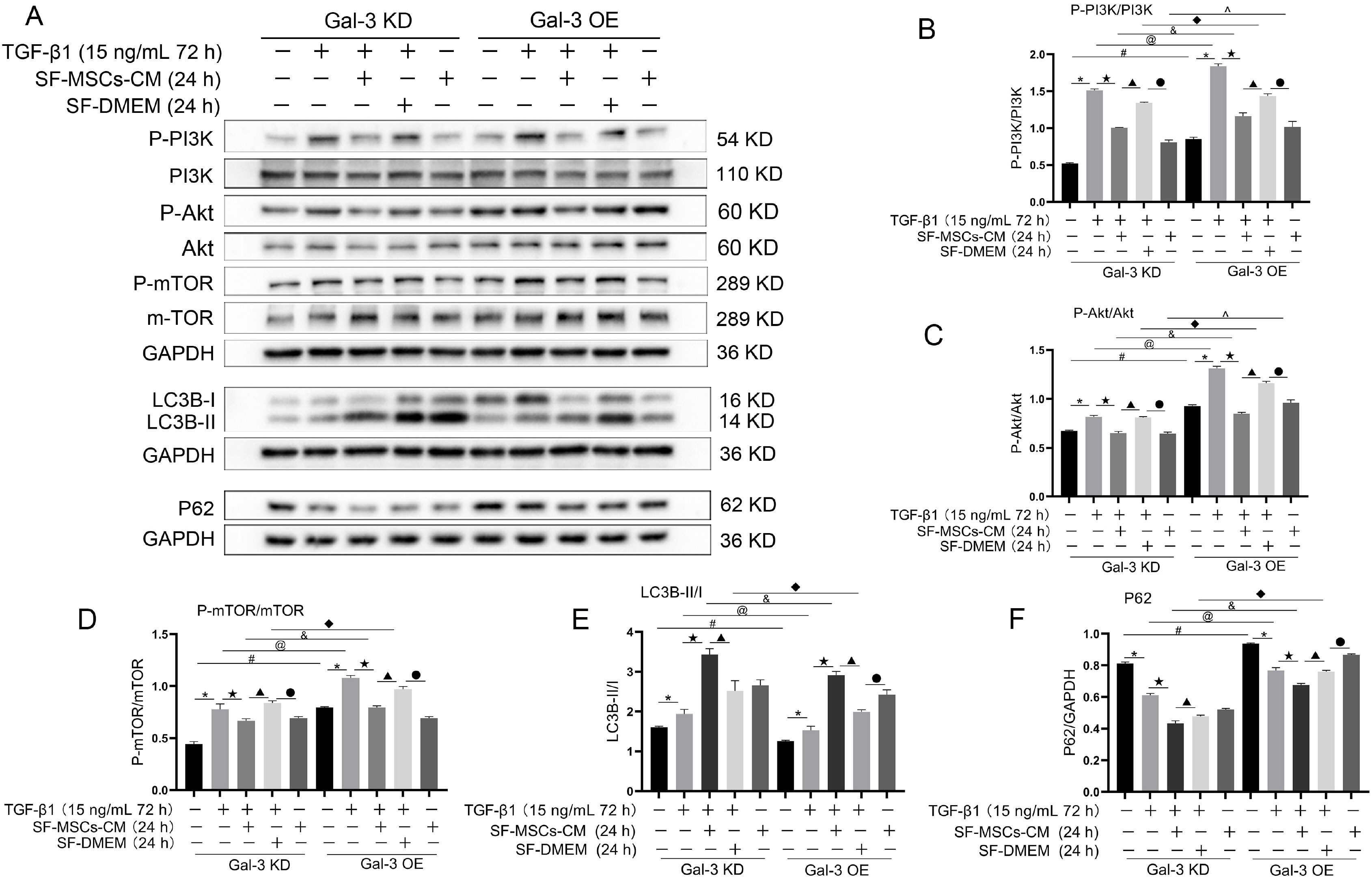
Galectin-3 knockdown (Gal-3 KD) and overexpression (Gal-3 OE) lentiviral vectors were established and transfected into NRK-52E. NRK-52E fibrosis model was induced by TGF-β1 and treated with the SF-MSCs-CM for 24 h after modelling. Fibrosis and autophagy related indexes were detected by western blot and immunocytochemistry. In model group, the expressions of α-smooth muscle actin (α-SMA), fibronectin (FN), Galectin-3, Snail, Kim-1, and the ratios of P-Akt/Akt, P-GSK3β/GSK3β, P-PI3K/PI3K, P-mTOR/mTOR, TIMP1/MMP9, and LC3B-II/I were obviously increased, and E-Cadherin (E-cad) and P62 decreased significantly compared with control group. SF-MSCs-CM showed an opposite trend after treatment compared with model group. Whether in Gal-3 KD or Gal-3 OE NRK-52E cells, SF-MSCs-CM also showed similar trends. However, the effects of anti-fibrosis and enhanced autophagy in Gal-3 KD cells were more obvious than those in Gal-3 OE cells.
Conclusions
SF-MSCs-CM probably alleviated the EMT via inhibiting Galectin-3/Akt/GSK3β/Snail pathway. Meanwhile, Gal-3 KD possibly enhanced autophagy via inhibiting Galectin-3/Akt/mTOR pathway, which synergistically ameliorated renal fibrosis. Targeting galectin-3 may be a potential target for the treatment of renal fibrosis.
Figure
Reference
-
References
1. Yan H, Xu J, Xu Z, Yang B, Luo P, He Q. 2021; Defining therapeutic targets for renal fibrosis: exploiting the biology of pathogenesis. Biomed Pharmacother. 143:112115. DOI: 10.1016/j.biopha.2021.112115. PMID: 34488081.2. Meng XM, Nikolic-Paterson DJ, Lan HY. 2016; TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 12:325–338. DOI: 10.1038/nrneph.2016.48. PMID: 27108839.3. Mack M, Yanagita M. 2015; Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 87:297–307. DOI: 10.1038/ki.2014.287. PMID: 25162398.4. Liu F, Zhuang S. 2019; New therapies for the treatment of renal fibrosis. Adv Exp Med Biol. 1165:625–659. DOI: 10.1007/978-981-13-8871-2_31. PMID: 31399988.5. Zhuang Q, Ma R, Yin Y, Lan T, Yu M, Ming Y. 2019; Mesenchymal stem cells in renal fibrosis: the flame of cytotherapy. Stem Cells Int. 2019:8387350. DOI: 10.1155/2019/8387350. PMID: 30766607. PMCID: PMC6350586.6. Prakoura N, Hadchouel J, Chatziantoniou C. 2019; Novel targets for therapy of renal fibrosis. J Histochem Cytochem. 67:701–715. DOI: 10.1369/0022155419849386. PMID: 31116064. PMCID: PMC6713972.7. Dikic I, Elazar Z. 2018; Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 19:349–364. DOI: 10.1038/s41580-018-0003-4. PMID: 29618831.8. Tang C, Livingston MJ, Liu Z, Dong Z. 2020; Autophagy in kidney homeostasis and disease. Nat Rev Nephrol. 16:489–508. DOI: 10.1038/s41581-020-0309-2. PMID: 32704047. PMCID: PMC7868042.9. Kim YA, Kim HJ, Gwon MG, Gu H, An HJ, Bae S, Leem J, Jung HJ, Park KK. 2021; Inhibitory effects of STAT3 transcription factor by synthetic decoy ODNs on autophagy in renal fibrosis. Biomedicines. 9:331. DOI: 10.3390/biomedicines9040331. PMID: 33806080. PMCID: PMC8064438. PMID: 7c9a1cc429ea4cb9abf73632804c6a7c.10. Xue X, Ren J, Sun X, Gui Y, Feng Y, Shu B, Wei W, Lu Q, Liang Y, He W, Yang J, Dai C. 2018; Protein kinase Cα drives fibroblast activation and kidney fibrosis by stimulating autophagic flux. J Biol Chem. 293:11119–11130. DOI: 10.1074/jbc.RA118.002191. PMID: 29794026. PMCID: PMC6052200.11. Gong W, Luo C, Peng F, Xiao J, Zeng Y, Yin B, Chen X, Li S, He X, Liu Y, Cao H, Xu J, Long H. 2021; Brahma-related gene-1 promotes tubular senescence and renal fibrosis through Wnt/β-catenin/autophagy axis. Clin Sci (Lond). 135:1873–1895. DOI: 10.1042/CS20210447. PMID: 34318888. PMCID: PMC8358963.12. Wang YJ, Chen YY, Hsiao CM, Pan MH, Wang BJ, Chen YC, Ho CT, Huang KC, Chen RJ. 2020; Induction of autophagy by pterostilbene contributes to the prevention of renal fibrosis via attenuating NLRP3 inflammasome activation and epithelial-mesenchymal transition. Front Cell Dev Biol. 8:436. DOI: 10.3389/fcell.2020.00436. PMID: 32582712. PMCID: PMC7283393. PMID: d5324ecb11604ed6978d463436dbb3da.13. Zhao XC, Livingston MJ, Liang XL, Dong Z. 2019; Cell apoptosis and autophagy in renal fibrosis. Adv Exp Med Biol. 1165:557–584. DOI: 10.1007/978-981-13-8871-2_28. PMID: 31399985.14. Gubert F, da Silva JS, Vasques JF, de Jesus Gonçalves RG, Martins RS, de Sá MPL, Mendez-Otero R, Zapata-Sudo G. 2021; Mesenchymal stem cells therapies on fibrotic heart diseases. Int J Mol Sci. 22:7447. DOI: 10.3390/ijms22147447. PMID: 34299066. PMCID: PMC8307175. PMID: f522e52b7c62449e9dc1b3cd6db5f7fa.15. Ntolios P, Steiropoulos P, Karpathiou G, Anevlavis S, Karampitsakos T, Bouros E, Froudarakis ME, Bouros D, Tzouvelekis A. 2020; Cell therapy for idiopathic pulmonary fibrosis: rationale and progress to date. BioDrugs. 34:543–556. DOI: 10.1007/s40259-020-00437-8. PMID: 32894503.16. Tsuchiya A, Takeuchi S, Watanabe T, Yoshida T, Nojiri S, Ogawa M, Terai S. 2019; Mesenchymal stem cell therapies for liver cirrhosis: MSCs as "conducting cells" for improvement of liver fibrosis and regeneration. Inflamm Regen. 39:18. DOI: 10.1186/s41232-019-0107-z. PMID: 31516638. PMCID: PMC6732839. PMID: 138c296deb904065b83df46c6ba220e2.17. Yun CW, Lee SH. 2019; Potential and therapeutic efficacy of cell-based therapy using mesenchymal stem cells for acute/chronic kidney disease. Int J Mol Sci. 20:1619. DOI: 10.3390/ijms20071619. PMID: 30939749. PMCID: PMC6479813.18. Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, Hosseini SE, Falah N, Abbasi F, Baharvand H, Aghdami N. 2018; Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: a safety study with 18 months of follow-up. Cytothe-rapy. 20:660–669. DOI: 10.1016/j.jcyt.2018.02.368. PMID: 29580865.19. Quimby JM, Webb TL, Habenicht LM, Dow SW. 2013; Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther. 4:48. DOI: 10.1186/scrt198. PMCID: PMC3707049. PMID: 23632128.20. Perico N, Casiraghi F, Remuzzi G. 2018; Clinical translation of mesenchymal stromal cell therapies in nephrology. J Am Soc Nephrol. 29:362–375. DOI: 10.1681/ASN.2017070781. PMID: 29191959. PMCID: PMC5791082.21. Li H, Rong P, Ma X, Nie W, Chen Y, Zhang J, Dong Q, Yang M, Wang W. 2020; Mouse umbilical cord mesenchymal stem cell paracrine alleviates renal fibrosis in diabetic nephropathy by reducing myofibroblast transdifferentiation and cell proliferation and upregulating MMPs in mesangial cells. J Diabetes Res. 2020:3847171. DOI: 10.1155/2020/3847171. PMID: 32455132. PMCID: PMC7222483.22. Liu B, Ding FX, Liu Y, Xiong G, Lin T, He DW, Zhang YY, Zhang DY, Wei GH. 2018; Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton). 23:728–736. DOI: 10.1111/nep.13099. PMID: 28667820.23. Chen L, Wang Y, Li S, Zuo B, Zhang X, Wang F, Sun D. 2020; Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics. 10:9425–9442. DOI: 10.7150/thno.43315. PMID: 32802201. PMCID: PMC7415791.24. Bernard M, Yang B, Migneault F, Turgeon J, Dieudé M, Olivier MA, Cardin GB, El-Diwany M, Underwood K, Rodier F, Hébert MJ. 2020; Autophagy drives fibroblast senescence through MTORC2 regulation. Autophagy. 16:2004–2016. DOI: 10.1080/15548627.2020.1713640. PMID: 31931659. PMCID: PMC7595590.25. Shu S, Wang H, Zhu J, Liu Z, Yang D, Wu W, Cai J, Chen A, Tang C, Dong Z. 2021; Reciprocal regulation between ER stress and autophagy in renal tubular fibrosis and apoptosis. Cell Death Dis. 12:1016. DOI: 10.1038/s41419-021-04274-7. PMID: 34716302. PMCID: PMC8556380. PMID: 6d66ef031aac4178a31899264c356066.26. Li S, Lin Q, Shao X, Zhu X, Wu J, Wu B, Zhang M, Zhou W, Zhou Y, Jin H, Zhang Z, Qi C, Shen J, Mou S, Gu L, Ni Z. 2020; Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruc-tion. Free Radic Biol Med. 152:632–649. DOI: 10.1016/j.freeradbiomed.2019.12.005. PMID: 31825802.27. Kimura T, Takahashi A, Takabatake Y, Namba T, Yamamoto T, Kaimori JY, Matsui I, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y. 2013; Autophagy protects kidney proximal tubule epithelial cells from mitochondrial metabolic stress. Autophagy. 9:1876–1886. DOI: 10.4161/auto.25418. PMID: 24128672.28. Chen K, Yu B, Liao J. 2021; LncRNA SOX2OT alleviates mesangial cell proliferation and fibrosis in diabetic nephropathy via Akt/mTOR-mediated autophagy. Mol Med. 27:71. DOI: 10.1186/s10020-021-00310-6. PMCID: PMC8268185. PMID: 34238205. PMID: e0f6f6d7e06e4f58a37af1c528ab3121.29. Cong LH, Li T, Wang H, Wu YN, Wang SP, Zhao YY, Zhang GQ, Duan J. 2020; IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/Akt/mTOR-mediated autophagy. J Cell Mol Med. 24:8532–8544. DOI: 10.1111/jcmm.15475. PMID: 32643865. PMCID: PMC7412687.30. Dai J, Sun Y, Chen D, Zhang Y, Yan L, Li X, Wang J. 2019; Negative regulation of PI3K/AKT/mTOR axis regulates fibroblast proliferation, apoptosis and autophagy play a vital role in triptolide-induced epidural fibrosis reduction. Eur J Pharmacol. 864:172724. DOI: 10.1016/j.ejphar.2019.172724. PMID: 31600493.31. Nam SA, Kim WY, Kim JW, Park SH, Kim HL, Lee MS, Komatsu M, Ha H, Lim JH, Park CW, Yang CW, Kim J, Kim YK. 2019; Autophagy attenuates tubulointerstital fibrosis through regulating transforming growth factor-β and NLRP3 inflammasome signaling pathway. Cell Death Dis. 10:78. DOI: 10.1038/s41419-019-1356-0. PMID: 30692509. PMCID: PMC6349890.32. Zhao XC, Livingston MJ, Liang XL, Dong Z. 2019; Cell apoptosis and autophagy in renal fibrosis. Adv Exp Med Biol. 1165:557–584. DOI: 10.1007/978-981-13-8871-2_28. PMID: 31399985.33. Saccon F, Gatto M, Ghirardello A, Iaccarino L, Punzi L, Doria A. 2017; Role of galectin-3 in autoimmune and non-autoimmune nephropathies. Autoimmun Rev. 16:34–47. DOI: 10.1016/j.autrev.2016.09.023. PMID: 27666815.34. Hara A, Niwa M, Noguchi K, Kanayama T, Niwa A, Matsuo M, Hatano Y, Tomita H. 2020; Galectin-3 as a next-generation biomarker for detecting early stage of various diseases. Biomolecules. 10:389. DOI: 10.3390/biom10030389. PMID: 32138174. PMCID: PMC7175224. PMID: d4cf0ba4c34c41ef866598d2dfcf61e0.35. Martínez-Martínez E, Ibarrola J, Fernández-Celis A, Calvier L, Leroy C, Cachofeiro V, Rossignol P, López-Andrés N. 2018; Galectin-3 pharmacological inhibition attenuates early renal damage in spontaneously hypertensive rats. J Hypertens. 36:368–376. DOI: 10.1097/HJH.0000000000001545. PMID: 28858976.36. Oikonomou T, Goulis I, Ntogramatzi F, Athanasiadou Z, Vagdatli E, Akriviadis E, Cholongitas E. 2019; Galectin-3 is associated with glomerular filtration rate and outcome in patients with stable decompensated cirrhosis. Dig Liver Dis. 51:1692–1697. DOI: 10.1016/j.dld.2019.05.030. PMID: 31235313.37. Shen H, Wang J, Min J, Xi W, Gao Y, Yin L, Yu Y, Liu K, Xiao J, Zhang YF, Wang ZN. 2018; Activation of TGF-β1/α-SMA/Col I profibrotic pathway in fibroblasts by galectin-3 contributes to atrial fibrosis in experimental models and patients. Cell Physiol Biochem. 47:851–863. DOI: 10.1159/000490077. PMID: 29807358.38. Dong R, Zhang M, Hu Q, Zheng S, Soh A, Zheng Y, Yuan H. 2018; Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med. 41:599–614. DOI: 10.3892/ijmm.2017.3311. PMID: 29207027. PMCID: PMC5752178.39. Wang Y, Chen X, Cao W, Shi Y. 2014; Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 15:1009–1016. DOI: 10.1038/ni.3002. PMID: 25329189.40. Yang D, Wang W, Li L, Peng Y, Chen P, Huang H, Guo Y, Xia X, Wang Y, Wang H, Wang WE, Zeng C. 2013; The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS One. 8:e59020. DOI: 10.1371/journal.pone.0059020. PMCID: PMC3602597. PMID: 23527076. PMID: 0c2856f8d3ba41a995332f455e19b3bd.41. d'Angelo M, Cimini A, Castelli V. 2020; Insights into the effects of mesenchymal stem cell-derived secretome in Parkinson's disease. Int J Mol Sci. 21:5241. DOI: 10.3390/ijms21155241. PMID: 32718092. PMCID: PMC7432166. PMID: f08f081b48a646f3a76ce03d25a2ac72.42. Gunawardena TNA, Rahman MT, Abdullah BJJ, Abu Kasim NH. 2019; Conditioned media derived from mesenchymal stem cell cultures: the next generation for regenerative medicine. J Tissue Eng Regen Med. 13:569–586. DOI: 10.1002/term.2806. PMID: 30644175.43. Kholia S, Herrera Sanchez MB, Cedrino M, Papadimitriou E, Tapparo M, Deregibus MC, Bruno S, Antico F, Brizzi MF, Quesenberry PJ, Camussi G. 2020; Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front Cell Dev Biol. 8:188. DOI: 10.3389/fcell.2020.00188. PMID: 32266268. PMCID: PMC7105599. PMID: 30db69ecc4864a438e8049a7860729bc.44. Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. 2012; Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 7:e44092. DOI: 10.1371/journal.pone.0044092. PMID: 22970165. PMCID: PMC3435420. PMID: 565ef4ee4e9145c78eb21cf4b072b0c6.45. Yu B, Zhang X, Li X. 2014; Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 15:4142–4157. DOI: 10.3390/ijms15034142. PMID: 24608926. PMCID: PMC3975389.46. Yin S, Zhou S, Ren D, Zhang J, Xin H, He X, Gao H, Hou J, Zeng F, Lu Y, Zhang X, Fan M. 2022; Mesenchymal stem cell-derived exosomes attenuate epithelial-mesenchymal transition of HK-2 cells. Tissue Eng Part A. doi: 10.1089/ten.TEA.2021.0190. [Epub ahead of print]. DOI: 10.1089/ten.tea.2021.0190. PMID: 35019728.47. Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, Brennan EP, Wilkinson-Berka JL, Wise AF, Ricardo SD. 2016; Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 24:1290–1301. DOI: 10.1038/mt.2016.90. PMID: 27203438. PMCID: PMC5088767.48. Shi Z, Wang Q, Zhang Y, Jiang D. 2020; Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem Cell Res Ther. 11:253. DOI: 10.1186/s13287-020-01767-8. PMID: 32586368. PMCID: PMC7318505. PMID: ffa4bded8b22465c881a608e1765d922.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-21 promotes epithelial-mesenchymal transition and migration of human bronchial epithelial cells by targeting poly (ADP-ribose) polymerase-1 and activating PI3K/AKT signaling
- Melatonin Rescues Mesenchymal Stem Cells from Senescence Induced by the Uremic Toxin p-Cresol via Inhibiting mTOR-Dependent Autophagy
- Extracellular Ubiquitin Enhances Autophagy and Inhibits Mitochondrial Apoptosis Pathway to Protect Neurons Against Spinal Cord Ischemic Injury via CXCR4
- The Role of Epithelial-mesenchymal Transition in the Gastroenterology
- Dasatinib induces apoptosis and autophagy by suppressing the PI3K/Akt/mTOR pathway in bladder cancer cells