Korean J Gastroenterol.
2023 Feb;81(2):86-90. 10.4166/kjg.2023.002.
Diagnosis and Treatment of Primary Biliary Cholangitis
- Affiliations
-
- 1Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea
- KMID: 2539577
- DOI: http://doi.org/10.4166/kjg.2023.002
Abstract
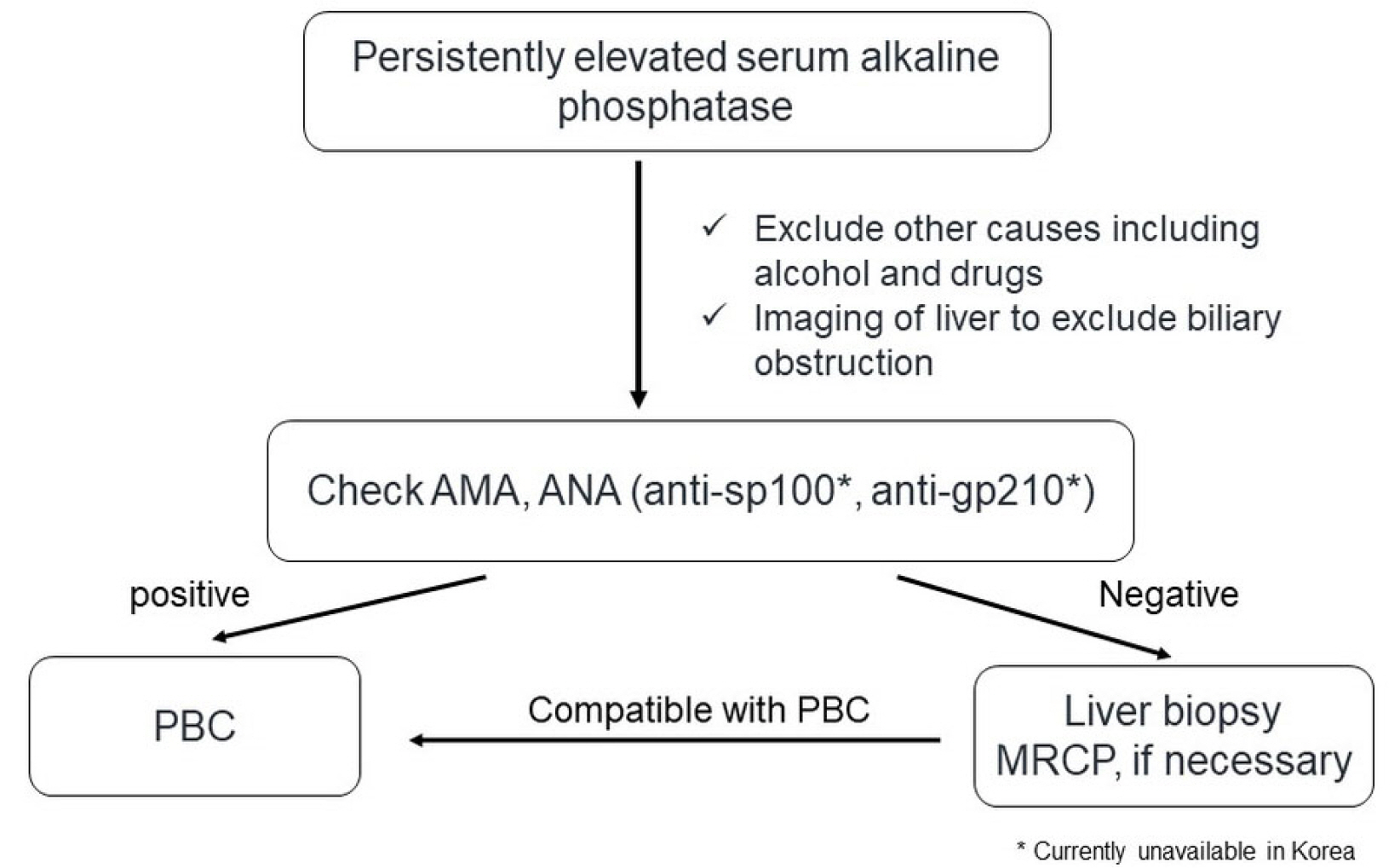
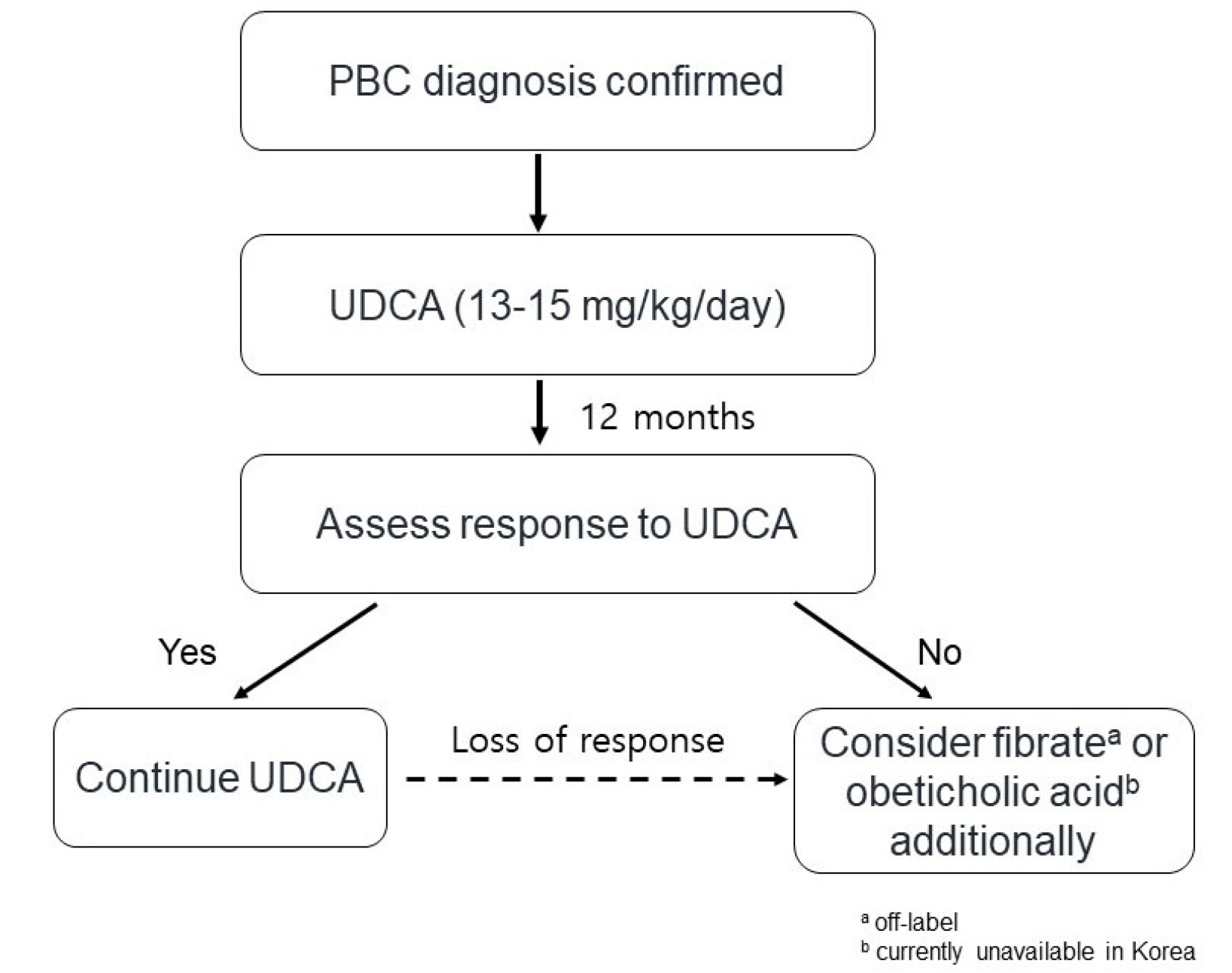
- Primary biliary cholangitis (PBC) is an autoimmune disease prevalent in middle-aged women and characterized by chronic cholestasis. PBC is diagnosed when at least two of the following three criteria are met: elevated alkaline phosphatase, presence of PBC-specific autoantibodies such as the anti-mitochondrial antibody or PBC-specific anti-nuclear antibodies, and non-suppurative inflammation of the interlobular bile duct after excluding other causes including drugs and biliary obstruction. The first-line treatment for PBC is ursodeoxycholic acid (UDCA, 13-15 mg/kg/day). The response to UDCA is predictive of long-term prognosis and should be evaluated 6-12 months after the UDCA treatment. The second-line treatments for PBC recommended due to an inadequate response to UDCA include obeticholic acid and fibrates. Symptoms and complications, including pruritus, sicca syndrome, and osteoporosis, should be evaluated and appropriately managed.
Keyword
Figure
Reference
-
1. Kaplan MM. 1996; Primary biliary cirrhosis. N Engl J Med. 335:1570–1580. DOI: 10.1056/NEJM199611213352107. PMID: 8900092.
Article2. Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. 2021; Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 36:1423–1434. DOI: 10.1111/jgh.15329. PMID: 33141955.
Article3. Kim KA, Ki M, Choi HY, Kim BH, Jang ES, Jeong SH. 2016; Populationbased epidemiology of primary biliary cirrhosis in South Korea. Aliment Pharmacol Ther. 43:154–162. DOI: 10.1111/apt.13448. PMID: 26526639.
Article4. Kim KA, Choi HY, Jang ES, Ki M, Jeong SH. 2022; Population-based epidemiology and outcomes of primary biliary cholangitis in South Korea from 2009 to 2019. Hepatol Int. 16(Suppl 1):S300. Abstract no. PP-1060.5. Cordell HJ, Fryett JJ, Ueno K, et al. PBC Consortia. Chinese PBC Consortium. Italian PBC Study Group. Japan-PBC-GWAS Consortium. US PBC Consortium. UK-PBC Consortium. An international genome-wide meta-analysis of primary biliary cholangitis: Novel risk loci and candidate drugs. J Hepatol. 2021; 75:572–581. Erratum in: J Hepatol 2022;76:489. Erratum in: J Hepatol. 2023 Jan 11. doi: 10.1016/j.jhep.2022.12.001.6. Younossi ZM, Bernstein D, Shiffman ML, et al. 2019; Diagnosis and management of primary biliary cholangitis. Am J Gastroenterol. 114:48–63. DOI: 10.1038/s41395-018-0390-3. PMID: 30429590.
Article7. Chen R, Tang R, Ma X, Gershwin ME. 2022; Immunologic responses and the pathophysiology of primary biliary cholangitis. Clin Liver Dis. 26:583–611. DOI: 10.1016/j.cld.2022.06.003. PMID: 36270718.
Article8. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. 2019; Primary biliary cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 69:394–419. DOI: 10.1002/hep.30145. PMID: 30070375.
Article9. European Association for the Study of the Liver. 2017; EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 67:145–172. DOI: 10.1016/j.jhep.2017.03.022. PMID: 28427765.10. Chen S, Duan WJ, You H, Ma X, Jia JD. 2022; [Recommendations of APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis]. Zhonghua Gan Zang Bing Za Zhi. 30:196–198. Chinese.11. Granito A, Muratori P, Muratori L, et al. 2006; Antinuclear antibodies giving the 'multiple nuclear dots' or the 'rim-like/membranous' patterns: diagnostic accuracy for primary biliary cirrhosis. Aliment Pharmacol Ther. 24:1575–1583. DOI: 10.1111/j.1365-2036.2006.03172.x. PMID: 17206945.
Article12. Nakamura M, Kondo H, Mori T, et al. 2007; Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 45:118–127. DOI: 10.1002/hep.21472. PMID: 17187436.
Article13. Angulo P, Lindor KD, Therneau TM, et al. 1999; Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver. 19:115–121. DOI: 10.1111/j.1478-3231.1999.tb00020.x. PMID: 10220741.
Article14. Azemoto N, Abe M, Murata Y, et al. 2009; Early biochemical response to ursodeoxycholic acid predicts symptom development in patients with asymptomatic primary biliary cirrhosis. J Gastroenterol. 44:630–634. DOI: 10.1007/s00535-009-0051-9. PMID: 19370305.
Article15. Corpechot C, Abenavoli L, Rabahi N, et al. 2008; Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 48:871–877. DOI: 10.1002/hep.22428. PMID: 18752324.
Article16. Parés A, Caballería L, Rodés J. 2006; Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 130:715–720. DOI: 10.1053/j.gastro.2005.12.029. PMID: 16530513.
Article17. Kuiper EM, Hansen BE, de Vries RA, et al. Dutch PBC Study Group. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009; 136:1281–1287. DOI: 10.1053/j.gastro.2009.01.003. PMID: 19208346.
Article18. Corpechot C, Chazouillères O, Poupon R. 2011; Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 55:1361–1367. DOI: 10.1016/j.jhep.2011.02.031. PMID: 21703194.
Article19. Kumagi T, Guindi M, Fischer SE, et al. 2010; Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 105:2186–2194. DOI: 10.1038/ajg.2010.216. PMID: 20502446.
Article20. Lammers WJ, Hirschfield GM, Corpechot C, et al. Global PBC Study Group. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015; 149:1804–1812.e4. DOI: 10.1053/j.gastro.2015.07.061. PMID: 26261009.
Article21. Carbone M, Sharp SJ, Flack S, et al. UK-PBC Consortium. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016; 63:930–950. DOI: 10.1002/hep.28017. PMID: 26223498. PMCID: PMC6984963.
Article22. Corpechot C, Chazouillères O, Rousseau A, et al. 2018; A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 378:2171–2181. DOI: 10.1056/NEJMoa1714519. PMID: 29874528.
Article23. Grigorian AY, Mardini HE, Corpechot C, Poupon R, Levy C. 2015; Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol. 39:296–306. DOI: 10.1016/j.clinre.2015.02.011. PMID: 25882906.
Article24. Laschtowitz A, de Veer RC, Van der Meer AJ, Schramm C. 2020; Diagnosis and treatment of primary biliary cholangitis. United European Gastroenterol J. 8:667–674. DOI: 10.1177/2050640620919585. PMID: 32299307. PMCID: PMC7437077.
Article25. Nevens F, Andreone P, Mazzella G, et al. POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016; 375:631–643. DOI: 10.1056/NEJMoa1509840. PMID: 27532829.
Article26. Leuschner M, Maier KP, Schlichting J, et al. 1999; Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 117:918–925. DOI: 10.1016/S0016-5085(99)70351-3. PMID: 10500075.
Article27. Hirschfield GM, Beuers U, Kupcinskas L, et al. 2021; A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA. J Hepatol. 74:321–329. DOI: 10.1016/j.jhep.2020.09.011. PMID: 32950590.
Article28. Montano-Loza AJ, Hansen BE, Corpechot C, et al. Global PBC Study Group. Factors associated with recurrence of primary biliary cholangitis after liver transplantation and effects on graft and patient survival. Gastroenterology. 2019; 156:96–107.e1. Erratum in: Gastroenterology 2020;158:288. DOI: 10.1053/j.gastro.2018.11.057. PMID: 30472231.
Article29. Bosch A, Dumortier J, Maucort-Boulch D, et al. 2015; Preventive administration of UDCA after liver transplantation for primary biliary cirrhosis is associated with a lower risk of disease recurrence. J Hepatol. 63:1449–1458. DOI: 10.1016/j.jhep.2015.07.038. PMID: 26282232.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biliary Tract & Pancreas; A Case of Cholangiocarcinoma Suggested as Developing in the Patient with Primary Sclerosing Cholangitis
- Epidemiology of Autoimmune Liver Disease
- Primary Sclerosing Cholangitis: One Case Report
- Primary Biliary Cholangitis with Ankylosing Spondylitis
- Two Cases of Primary Sclerosing Cholangitis