Brain Tumor Res Treat.
2023 Jan;11(1):8-15. 10.14791/btrt.2022.0045.
Molecular Biology of Brain Metastases
- Affiliations
-
- 1Department of Cancer Control, National Cancer Center, Graduate School of Cancer Science and Policy. National Cancer Center, Goyang, Korea
- KMID: 2539324
- DOI: http://doi.org/10.14791/btrt.2022.0045
Abstract
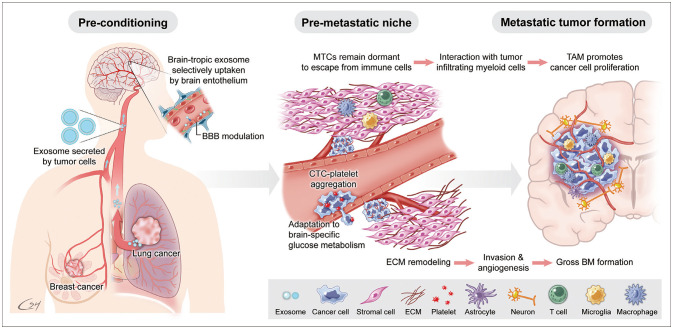
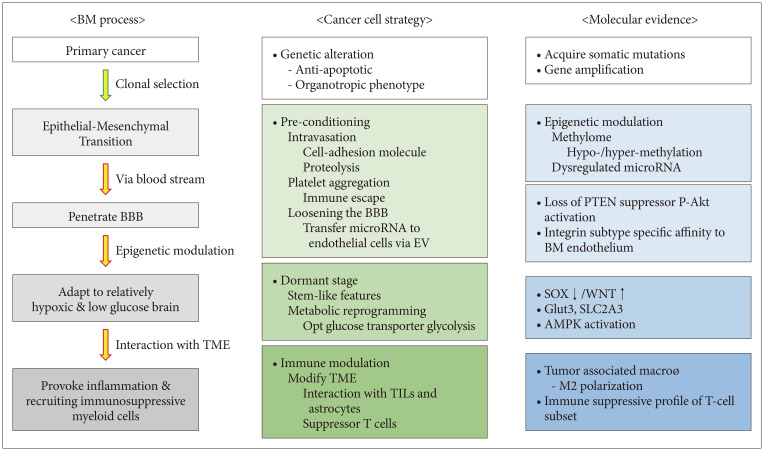
- Brain metastases (BMs) often occur in patients with lung cancer, breast cancer, and melanoma and are the leading cause of morbidity and mortality. The incidence of BM has increased with advanced neuroimaging and prolonged overall survival of cancer patients. With the advancement of local treatment modalities, including stereotactic radiosurgery and navigation-guided microsurgery, BM can be controlled long-term, even in cases with multiple lesions. However, radiation/chemotherapeutic agents are also toxic to the brain, usually irreversibly and cumulatively, and it remains difficult to completely cure BM. Thus, we must understand the molecular events that begin and sustain BM to develop effective targeted therapies and tools to prevent local and distant treatment failure. BM most often spreads hematogenously, and the blood–brain barrier (BBB) presents the first hurdle for disseminated tumor cells (DTCs) entering the brain parenchyma. Nevertheless, how the DTCs cross the BBB and settle on relatively infertile central nervous system tissue remains unknown. Even after successfully taking up residence in the brain, the unique tumor microenvironment is marked by restricted aerobic glycolysis metabolism and limited lymphocyte infiltration. Brain organotropism, certain phenotype of primary cancers that favors brain metastasis, may result from somatic mutation or epigenetic modulation. Recent studies revealed that exosome secretion from primary cancer or over-expression of proteolytic enzymes can “pre-condition” brain vasculoendothelial cells. The concept of the “metastatic niche,” where resident DTCs remain dormant and protected from systemic chemotherapy and antigen exposure before proliferation, is supported by clinical observation of BM in patients clearing systemic cancer and experimental evidence of the interaction between cancer cells and tumor-infiltrating lymphocytes. This review examines extant research on the metastatic cascade of BM through the molecular events that create and sustain BM to reveal clues that can assist the development of effective targeted therapies that treat established BMs and prevent BM recurrence.
Keyword
Figure
Reference
-
1. Adler JR, Cox RS, Kaplan I, Martin DP. Stereotactic radiosurgical treatment of brain metastases. J Neurosurg. 1992; 76:444–449. PMID: 1738025.
Article2. Alexander E 3rd, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995; 87:34–40. PMID: 7666461.
Article4. Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005; 167:913–920. PMID: 16192626.
Article5. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015; 88:108–111. PMID: 25682925.
Article6. Gwak HS, Yoo HJ, Youn SM, Lee DH, Kim MS, Rhee CH. Radiosurgery for recurrent brain metastases after whole-brain radiotherapy: factors affecting radiation-induced neurological dysfunction. J Korean Neurosurg Soc. 2009; 45:275–283. PMID: 19516944.
Article7. Hillard VH, Shih LL, Chin S, Moorthy CR, Benzil DL. Safety of multiple stereotactic radiosurgery treatments for multiple brain lesions. J Neurooncol. 2003; 63:271–278. PMID: 12892233.8. Yuzhalin AE, Yu D. Brain metastasis organotropism. Cold Spring Harb Perspect Med. 2020; 10:a037242. PMID: 31548224.9. Tadros S, Ray-Chaudhury A. Pathological features of brain metastases. Neurosurg Clin N Am. 2020; 31:549–564. PMID: 32921351.
Article10. Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003; 3:537–549. PMID: 12842083.
Article11. Basnet H, Tian L, Ganesh K, Huang YH, Macalinao DG, Brogi E, et al. Flura-seq identifies organ-specific metabolic adaptations during early metastatic colonization. Elife. 2019; 8:e43627. PMID: 30912515.
Article12. Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015; 527:100–104. PMID: 26479035.13. Jin Y, Yuan Y, Yi M, Han H, Liu B, Li Q. Phosphorylated-Akt overexpression is associated with a higher risk of brain metastasis in patients with non-small cell lung cancer. Biochem Biophys Rep. 2019; 18:100625. PMID: 30976664.14. Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol. 2014; 16:876–888. PMID: 25086747.15. Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, et al. The evolutionary history of 2,658 cancers. Nature. 2020; 578:122–128. PMID: 32025013.16. Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, et al. Integrative clinical genomics of metastatic cancer. Nature. 2017; 548:297–303. PMID: 28783718.17. Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015; 5:1164–1177. PMID: 26410082.18. Shih DJH, Nayyar N, Bihun I, Dagogo-Jack I, Gill CM, Aquilanti E, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020; 52:371–377. PMID: 32203465.19. Jacob LS, Vanharanta S, Obenauf AC, Pirun M, Viale A, Socci ND, et al. Metastatic competence can emerge with selection of preexisting oncogenic alleles without a need of new mutations. Cancer Res. 2015; 75:3713–3719. PMID: 26208905.20. Hamilton A, Sibson NR. Role of the systemic immune system in brain metastasis. Mol Cell Neurosci. 2013; 53:42–51. PMID: 23073146.21. Liu H, Chen J, Chen H, Xia J, Wang O, Xie J, et al. Identification of the origin of brain metastases based on the relative methylation orderings of CpG sites. Epigenetics. 2021; 16:908–916. PMID: 32965167.22. Salhia B, Kiefer J, Ross JT, Metapally R, Martinez RA, Johnson KN, et al. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS One. 2014; 9:e85448. PMID: 24489661.23. Melo SA, Esteller M. Dysregulation of microRNAs in cancer: playing with fire. FEBS Lett. 2011; 585:2087–2099. PMID: 20708002.24. Subramani A, Alsidawi S, Jagannathan S, Sumita K, Sasaki AT, Aronow B, et al. The brain microenvironment negatively regulates miRNA-768-3p to promote K-ras expression and lung cancer metastasis. Sci Rep. 2013; 3:2392. PMID: 23928793.25. Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014; 25:501–515. PMID: 24735924.26. Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002; 3:425–430. PMID: 12142172.27. Coupland LA, Parish CR. Platelets, selectins, and the control of tumor metastasis. Semin Oncol. 2014; 41:422–434. PMID: 25023359.28. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011; 20:576–590. PMID: 22094253.29. Erpenbeck L, Schön MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010; 115:3427–3436. PMID: 20194899.30. Kwon JW, Im JH, Lee KY, Yoo BC, Lee JH, Kim KH, et al. Different metabolomic and proteomic profiles of cerebrospinal fluid in ventricular and lumbar compartments in relation to leptomeningeal metastases. Metabolites. 2022; 12:80. PMID: 35050202.31. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005; 438:820–827. PMID: 16341007.32. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015; 527:329–335. PMID: 26524530.33. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014; 14:611–622. PMID: 25118602.34. Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016; 165:45–60. PMID: 27015306.35. Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci. 2013; 16:1373–1382. PMID: 23995067.36. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004; 4:891–899. PMID: 15516961.37. Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007; 67:1472–1486. PMID: 17308085.38. Lorger M. Tumor microenvironment in the brain. Cancers (Basel). 2012; 4:218–243. PMID: 24213237.39. Valiente M, Ahluwalia MS, Boire A, Brastianos PK, Goldberg SB, Lee EQ, et al. The evolving landscape of brain metastasis. Trends Cancer. 2018; 4:176–196. PMID: 29506669.40. Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014; 156:1002–1016. PMID: 24581498.41. Stevens LE, Cheung WKC, Adua SJ, Arnal-Estapé A, Zhao M, Liu Z, et al. Extracellular matrix receptor expression in subtypes of lung adenocarcinoma potentiates outgrowth of micrometastases. Cancer Res. 2017; 77:1905–1917. PMID: 28196904.42. Srinivasan ES, Deshpande K, Neman J, Winkler F, Khasraw M. The microenvironment of brain metastases from solid tumors. Neurooncol Adv. 2021; 3(Suppl 5):v121–v132. PMID: 34859239.43. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017; 31:326–341. PMID: 28292436.44. Schwartz H, Blacher E, Amer M, Livneh N, Abramovitz L, Klein A, et al. Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Res. 2016; 76:4359–4371. PMID: 27261506.45. Vilariño N, Bruna J, Bosch-Barrera J, Valiente M, Nadal E. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat Rev. 2020; 89:102067. PMID: 32682248.46. Mansfield AS, Ren H, Sutor S, Sarangi V, Nair A, Davila J, et al. Contraction of T cell richness in lung cancer brain metastases. Sci Rep. 2018; 8:2171. PMID: 29391594.47. Qiao S, Qian Y, Xu G, Luo Q, Zhang Z. Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J Neuroinflammation. 2019; 16:4. PMID: 30616691.48. Gonzalez H, Mei W, Robles I, Hagerling C, Allen BM, Hauge Okholm TL, et al. Cellular architecture of human brain metastases. Cell. 2022; 185:729–745.e20. PMID: 35063085.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in Understanding the Molecular Biology of Brain Tumors
- Radiomics and Deep Learning in Brain Metastases: Current Trends and Roadmap to Future Applications
- Predictive Factors and Survival Rate for Brain Metastasis from Breast Cancer
- Brain metastasis in human epidermal growth factor receptor 2-positive breast cancer: from biology to treatment
- Brain and Skull Metastases in Patients with Renal Cell Carcinoma: Follow-up Studies