J Korean Med Sci.
2023 Feb;38(5):e34. 10.3346/jkms.2023.38.e34.
Safety and Efficacy of Everolimus-Eluting Bioresorbable Vascular Scaffold Versus Second-Generation Drug-Eluting Stents in Real-World Practice
- Affiliations
-
- 1Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Cardiology, Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Korea
- 3Division of Cardiology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Division of Cardiology, Department of Internal Medicine, Bucheon Sejong Hospital, Bucheon, Korea
- 5Division of Cardiology, Department of Internal Medicine, Jeonbuk National University Hospital and Jeonbuk National University Medical School, Jeonju, Korea
- 6Division of Cardiology, Department of Internal Medicine, Keimyung University Dongsan Hospital, Daegu, Korea
- 7Division of Cardiology, Department of Internal Medicine, Chungbuk National University, Cheongju, Korea
- 8Division of Cardiology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 9Department of Cardiology, Pusan National University Yangsan Hospital, Yangsan, Korea
- 10Division of Cardiology, Department of Internal Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- 11Division of Cardiology, Department of Internal Medicine, Kangwon National University Hospital, Kangwon National University College of Medicine, Chuncheon, Korea
- 12Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA, USA
- 13Division of Cardiology, Department of Internal Medicine, Chonnam National University Hospital, Gwangju, Korea
- 14Department of Internal Medicine and Cardiovascular Center, Seoul National University Hospital, Seoul, Korea
- 15Department of Internal Medicine and Cardiovascular Center, Chosun University Hospital, University of Chosun College of Medicine, Gwangju, Korea
- 16Division of Cardiology, Department of Internal Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- 17Department of Cardiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 18Department of Cardiology, Inje University Ilsan Paik Hospital, Goyang, Korea
- KMID: 2539198
- DOI: http://doi.org/10.3346/jkms.2023.38.e34
Abstract
- Background
The risk of device thrombosis and device-oriented clinical outcomes with bioresorbable vascular scaffold (BVS) was reported to be significantly higher than with contemporary drug-eluting stents (DESs). However, optimal device implantation may improve clinical outcomes in patients receiving BVS. The current study evaluated mid-term safety and efficacy of Absorb BVS with meticulous device optimization under intravascular imaging guidance.
Methods
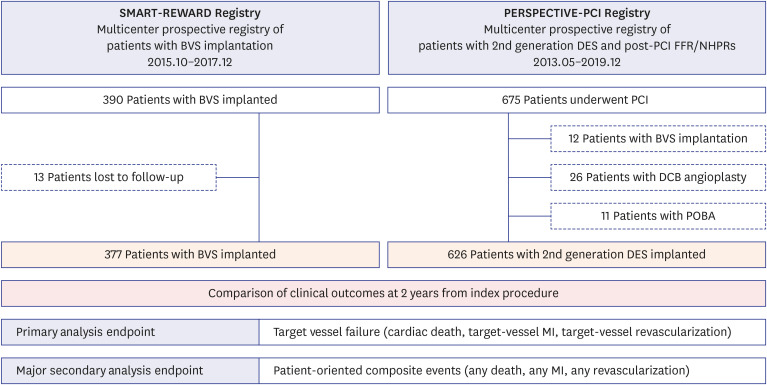
The SMART-REWARD and PERSPECTIVE-PCI registries in Korea prospectively enrolled 390 patients with BVS and 675 patients with DES, respectively. The primary endpoint was target vessel failure (TVF) at 2 years and the secondary major endpoint was patientoriented composite outcome (POCO) at 2 years.
Results
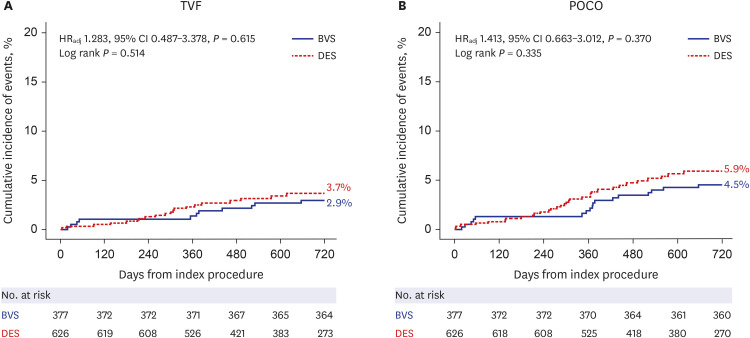
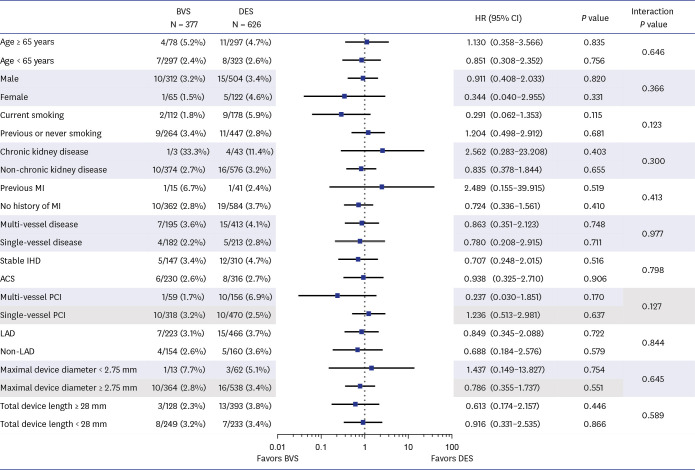
Patient-level pooled analysis evaluated 1,003 patients (377 patients with BVS and 626 patients with DES). Mean scaffold diameter per lesion was 3.24 ± 0.30 mm in BVS group. Most BVSs were implanted with pre-dilatation (90.9%), intravascular imaging guidance (74.9%), and post-dilatation (73.1%) at proximal to mid segment (81.9%) in target vessel. Patients treated with BVS showed comparable risks of 2-year TVF (2.9% vs. 3.7%, adjusted hazard ratio [HR], 1.283, 95% confidence interval [CI], 0.487–3.378, P = 0.615) and 2-year POCO (4.5% vs. 5.9%, adjusted HR, 1.413, 95% CI, 0.663–3.012,P = 0.370) than those with DES. The rate of 2-year definite or probable device thrombosis (0.3% vs. 0.5%, P = 0.424) was also similar. The sensitivity analyses consistently showed comparable risk of TVF and POCO between the 2 groups.
Conclusion
With meticulous device optimization under imaging guidance and avoidance of implantation in small vessels, BVS showed comparable risks of 2-year TVF and device thrombosis with DES.
Figure
Reference
-
1. Ahn JM, Park DW, Hong SJ, Ahn YK, Hahn JY, Kim WJ, et al. Bioresorbable vascular scaffold Korean expert panel report. Korean Circ J. 2017; 47(6):795–810. PMID: 29171214.2. Yamaji K, Kimura T, Morimoto T, Nakagawa Y, Inoue K, Soga Y, et al. Very long-term (15 to 20 years) clinical and angiographic outcome after coronary bare metal stent implantation. Circ Cardiovasc Interv. 2010; 3(5):468–475. PMID: 20823392.3. Onuma Y, Dudek D, Thuesen L, Webster M, Nieman K, Garcia-Garcia HM, et al. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv. 2013; 6(10):999–1009. PMID: 24156961.4. Serruys PW, Ormiston J, van Geuns RJ, de Bruyne B, Dudek D, Christiansen E, et al. A polylactide bioresorbable scaffold eluting everolimus for treatment of coronary stenosis: 5-year follow-up. J Am Coll Cardiol. 2016; 67(7):766–776. PMID: 26892411.5. Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, García-García HM, et al. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation. 2010; 122(22):2288–2300. PMID: 20975003.6. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrié D, Piek JJ, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016; 388(10059):2479–2491. PMID: 27806897.7. Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, et al. 3-year clinical outcomes with everolimus-eluting bioresorbable coronary scaffolds: the ABSORB III trial. J Am Coll Cardiol. 2017; 70(23):2852–2862. PMID: 29100702.8. Wykrzykowska JJ, Kraak RP, Hofma SH, van der Schaaf RJ, Arkenbout EK, IJsselmuiden AJ, et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. 2017; 376(24):2319–2328. PMID: 28402237.9. Ali ZA, Serruys PW, Kimura T, Gao R, Ellis SG, Kereiakes DJ, et al. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017; 390(10096):760–772. PMID: 28732815.10. Puricel S, Cuculi F, Weissner M, Schmermund A, Jamshidi P, Nyffenegger T, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol. 2016; 67(8):921–931. PMID: 26916481.11. Sotomi Y, Suwannasom P, Serruys PW, Onuma Y. Possible mechanical causes of scaffold thrombosis: insights from case reports with intracoronary imaging. EuroIntervention. 2017; 12(14):1747–1756. PMID: 27773862.12. Ellis SG, Gori T, Serruys PW, Nef H, Steffenino G, Brugaletta S, et al. Clinical, angiographic, and procedural correlates of very late absorb scaffold thrombosis: multistudy registry results. JACC Cardiovasc Interv. 2018; 11(7):638–644. PMID: 29622141.13. Ortega-Paz L, Capodanno D, Gori T, Nef H, Latib A, Caramanno G, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: development and internal validation of the PSP score. EuroIntervention. 2017; 12(17):2110–2117. PMID: 28246060.14. Shin D, Lee SH, Lee JM, Choi KH, Hwang D, Lee HJ, et al. Prognostic implications of post-intervention resting Pd/Pa and fractional flow reserve in patients with stent implantation. JACC Cardiovasc Interv. 2020; 13(16):1920–1933. PMID: 32819481.15. Shin D, Dai N, Lee SH, Choi KH, Lefieux A, Molony D, et al. Physiological distribution and local severity of coronary artery disease and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2021; 14(16):1771–1785. PMID: 34412795.16. Chang K, Ahn Y, Lim S, Yang JH, Lee KY, Choo EH, et al. 2021 Korean Society of Myocardial Infarction expert consensus document on revascularization for acute myocardial infarction. Korean Circ J. 2021; 51(4):289–307. PMID: 33821579.17. Park KH, Jeong MH, Kim HK, Ki YJ, Kim SS, Choi DH, et al. Clinical outcomes of ticagrelor in Korean patients with acute myocardial infarction without high bleeding risk. J Korean Med Sci. 2021; 36(42):e268. PMID: 34725976.18. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. 2018; 137(24):2635–2650. PMID: 29891620.19. Ellis SG, Kereiakes DJ, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015; 373(20):1905–1915. PMID: 26457558.20. Gao R, Yang Y, Han Y, Huo Y, Chen J, Yu B, et al. Bioresorbable Vascular Scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China trial. J Am Coll Cardiol. 2015; 66(21):2298–2309. PMID: 26471805.21. Kimura T, Kozuma K, Tanabe K, Nakamura S, Yamane M, Muramatsu T, et al. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J. 2015; 36(47):3332–3342. PMID: 26330419.22. Chevalier B, Onuma Y, van Boven AJ, Piek JJ, Sabaté M, Helqvist S, et al. Randomised comparison of a bioresorbable everolimus-eluting scaffold with a metallic everolimus-eluting stent for ischaemic heart disease caused by de novo native coronary artery lesions: the 2-year clinical outcomes of the ABSORB II trial. EuroIntervention. 2016; 12(9):1102–1107. PMID: 27564310.23. Waksman R. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. 2017; 377(18):1790–1791.24. Kereiakes DJ, Ellis SG, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, et al. Clinical outcomes before and after complete everolimus-eluting bioresorbable scaffold resorption: five-year follow-up from the ABSORB III trial. Circulation. 2019; 140(23):1895–1903. PMID: 31553222.25. Kerkmeijer LS, Renkens MP, Tijssen RY, Hofma SH, van der Schaaf RJ, Arkenbout EK, et al. Long-term clinical outcomes of everolimus-eluting bioresorbable scaffolds versus everolimus-eluting stents: final five-year results of the AIDA randomised clinical trial. EuroIntervention. 2022; 17(16):1340–1347. PMID: 34483094.26. Kozuma K, Tanabe K, Hamazaki Y, Okamura T, Ando J, Ikari Y, et al. Long-term outcomes of absorb bioresorbable vascular scaffold vs. everolimus-eluting metallic stent – A randomized comparison through 5 years in Japan. Circ J. 2020; 84(5):733–741. PMID: 32213737.27. Räber L, Brugaletta S, Yamaji K, O’Sullivan CJ, Otsuki S, Koppara T, et al. Very late scaffold thrombosis: intracoronary imaging and histopathological and spectroscopic findings. J Am Coll Cardiol. 2015; 66(17):1901–1914. PMID: 26493663.28. Yamaji K, Ueki Y, Souteyrand G, Daemen J, Wiebe J, Nef H, et al. Mechanisms of very late bioresorbable scaffold thrombosis: the INVEST registry. J Am Coll Cardiol. 2017; 70(19):2330–2344. PMID: 29096803.29. Jinnouchi H, Torii S, Sakamoto A, Kolodgie FD, Virmani R, Finn AV. Fully bioresorbable vascular scaffolds: lessons learned and future directions. Nat Rev Cardiol. 2019; 16(5):286–304. PMID: 30546115.30. Onuma Y, Serruys PW, Muramatsu T, Nakatani S, van Geuns RJ, de Bruyne B, et al. Incidence and imaging outcomes of acute scaffold disruption and late structural discontinuity after implantation of the absorb everolimus-eluting fully bioresorbable vascular scaffold: optical coherence tomography assessment in the ABSORB cohort B trial (a clinical evaluation of the bioabsorbable everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2014; 7(12):1400–1411. PMID: 25523532.31. Friedman MH. Variability of arterial wall shear stress, its dependence on vessel diameter and implications for Murray’s Law. Atherosclerosis. 2009; 203(1):47–48. PMID: 18715565.32. Okada K, Honda Y, Kitahara H, Otagiri K, Tanaka S, Hollak MB, et al. Bioresorbable scaffold for treatment of coronary artery lesions: intravascular ultrasound results from the ABSORB Japan trial. JACC Cardiovasc Interv. 2018; 11(7):648–661. PMID: 29622143.33. Taglieri N, Bruno AG, Ghetti G, Marrozzini C, Saia F, Galié N, et al. Target lesion failure with current drug-eluting stents: evidence from a comprehensive network meta-analysis. JACC Cardiovasc Interv. 2020; 13(24):2868–2878. PMID: 33357524.34. Madhavan MV, Howard JP, Naqvi A, Ben-Yehuda O, Redfors B, Prasad M, et al. Long-term follow-up after ultrathin vs. conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2021; 42(27):2643–2654. PMID: 34002202.35. Pilgrim T, Rothenbühler M, Siontis GC, Kandzari DE, Iglesias JF, Asami M, et al. Biodegradable polymer sirolimus-eluting stents vs durable polymer everolimus-eluting stents in patients undergoing percutaneous coronary intervention: a meta-analysis of individual patient data from 5 randomized trials. Am Heart J. 2021; 235:140–148. PMID: 33609498.36. Onuma Y, Sotomi Y, Shiomi H, Ozaki Y, Namiki A, Yasuda S, et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention. 2016; 12(9):1090–1101. PMID: 27597270.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bioresorbable Scaffolds in Coronary Intervention: Unmet Needs and Evolution
- Dark Side of Drug-eluting Stent in Contemporary Percutaneous Coronary Intervention
- Coronary Stent Thrombosis: Current Insights into New Drug-Eluting Stent Designs
- Stent Optimization Using Adjunctive Balloon Dilatation in the Era of Second-Generation Drug-Eluting Stents
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis