Diabetes Metab J.
2023 Jan;47(1):92-103. 10.4093/dmj.2021.0370.
Effect of Lactobacillus plantarum LMT1-48 on Body Fat in Overweight Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Medytox Inc., Seoul, Korea
- 3Medytox Gwangkyo R&D Center, Suwon, Korea
- KMID: 2538934
- DOI: http://doi.org/10.4093/dmj.2021.0370
Abstract
- Background
We investigated whether Lactobacillus plantarum strain LMT1-48, isolated from Korean fermented foods and newborn feces, is a suitable probiotic supplement to treat overweight subjects.
Methods
In this randomized, double-blind, placebo-controlled clinical trial, 100 volunteers with a body mass index of 25 to 30 kg/m2 were assigned randomly (1:1) to receive 2×1010 colony forming units of LMT1-48 or to a placebo treatment group. Body composition was measured by dual-energy X-ray absorptiometry, and abdominal visceral fat area (VFA) and subcutaneous fat area were measured by computed tomography scanning. Changes in body fat, VFA, anthropometric parameters, and biomarkers were compared between the two treatment groups (ClinicalTrials.gov number: NCT03759743).
Results
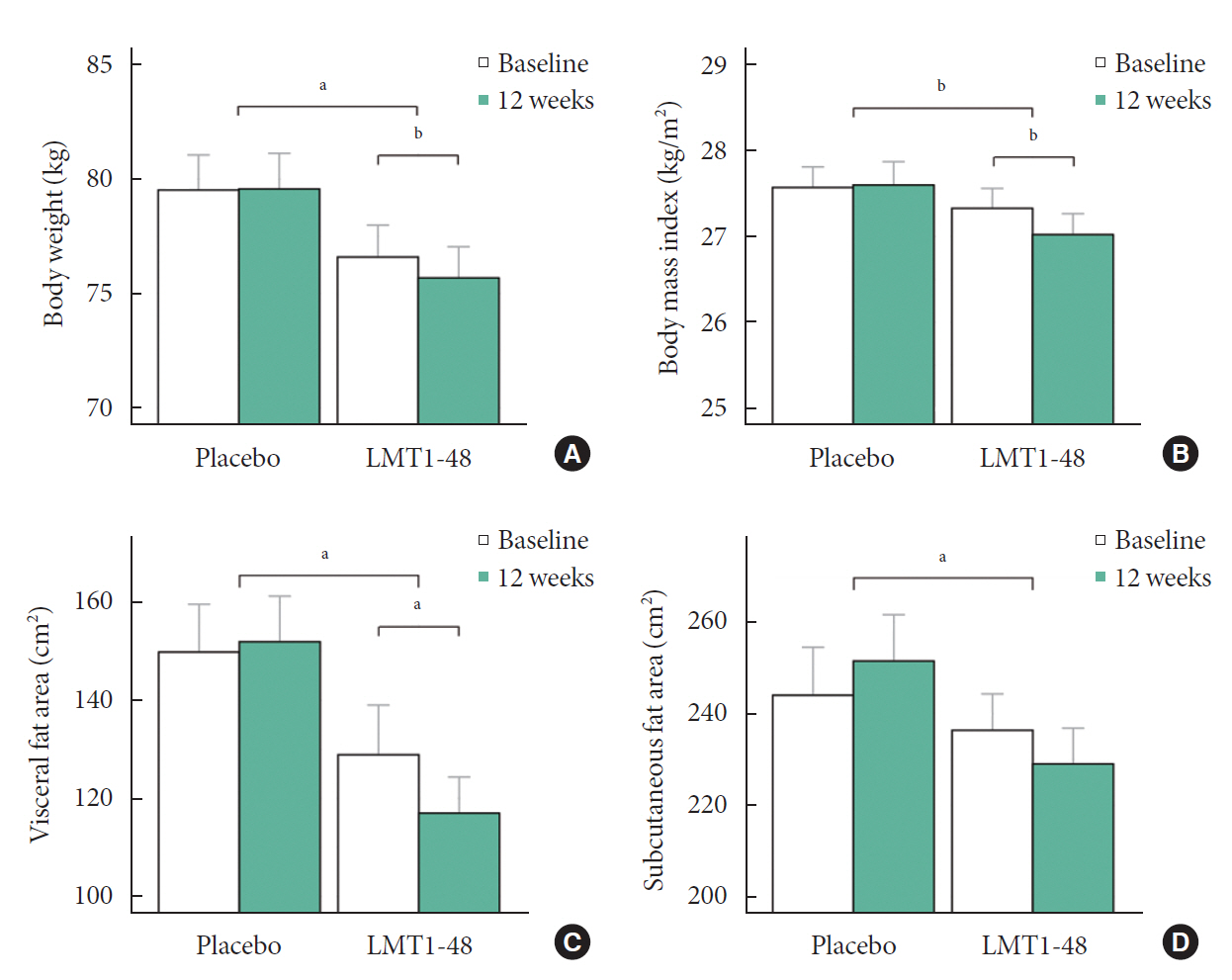
After 12 weeks of treatment, the body weight decreased significantly from 76.6±9.4 to 75.7±9.2 kg in the LMT1-48 group but did not change in the placebo group (P=0.022 between groups). A similar pattern was found in abdominal VFA between the two groups (P=0.041). Serum insulin levels, the corresponding homeostasis model assessment of insulin resistance, and leptin levels decreased in the LMT1-48 group but increased in the placebo group (all P<0.05). Decrease in body weight and body mass index by treatment with LMT1-48 was correlated with increase in Lactobacillus levels significantly. LMT1-48 also increased Oscillibacter levels significantly, which were negatively correlated with triglyceride and alanine transaminase levels.
Conclusion
Administration of LMT1-48 decreased body weight, abdominal VFA, insulin resistance, and leptin levels in these subjects with overweight, suggesting its anti-obesogenic therapeutic potential.
Figure
Reference
-
1. Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019; 15:288–98.
Article2. World Health Organization: Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (updated 2021 Jun 9).3. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012; 125:1157–70.4. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011; 121:2126–32.
Article5. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012; 489:242–9.
Article6. Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. 2020; 9:179–92.
Article7. Ejtahed HS, Angoorani P, Soroush AR, Atlasi R, Hasani-Ranjbar S, Mortazavian AM, et al. Probiotics supplementation for the obesity management; a systematic review of animal studies and clinical trials. J Funct Foods. 2019; 52:228–42.
Article8. Wicinski M, Gebalski J, Golebiewski J, Malinowski B. Probiotics for the treatment of overweight and obesity in humans: a review of clinical trials. Microorganisms. 2020; 8:1148.
Article9. Borgeraas H, Johnson LK, Skattebu J, Hertel JK, Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2018; 19:219–32.
Article10. Crovesy L, Ostrowski M, Ferreira DM, Rosado EL, Soares-Mota M. Effect of Lactobacillus on body weight and body fat in overweight subjects: a systematic review of randomized controlled clinical trials. Int J Obes (Lond). 2017; 41:1607–14.
Article11. Lim S, Moon JH, Shin CM, Jeong D, Kim B. Effect of Lactobacillus sakei, a probiotic derived from kimchi, on body fat in Koreans with obesity: a randomized controlled study. Endocrinol Metab (Seoul). 2020; 35:425–34.
Article12. Woodard GA, Encarnacion B, Downey JR, Peraza J, Chong K, Hernandez-Boussard T, et al. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009; 13:1198–204.
Article13. Liu YW, Liong MT, Tsai YC. New perspectives of Lactobacillus plantarum as a probiotic: the gut-heart-brain axis. J Microbiol. 2018; 56:601–13.
Article14. Kim B, Choi HN, Yim JE. Effect of diet on the gut microbiota associated with obesity. J Obes Metab Syndr. 2019; 28:216–24.
Article15. Choi WJ, Dong HJ, Jeong HU, Jung HH, Kim YH, Kim TH. Antiobesity effects of Lactobacillus plantarum LMT1-48 accompanied by inhibition of Enterobacter cloacae in the intestine of diet-induced obese mice. J Med Food. 2019; 22:560–6.16. Choi WJ, Dong HJ, Jeong HU, Ryu DW, Song SM, Kim YR, et al. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci Rep. 2020; 10:869.
Article17. Sohn M, Na GY, Chu J, Joung H, Kim BK, Lim S. Efficacy and safety of Lactobacillus plantarum K50 on lipids in Koreans with obesity: a randomized, double-blind controlled clinical trial. Front Endocrinol (Lausanne). 2022; 12:790046.
Article18. Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018; 34:i884–90.
Article19. Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011; 27:2957–63.
Article20. Li W, Fu L, Niu B, Wu S, Wooley J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief Bioinform. 2012; 13:656–68.
Article21. Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009; 10:421.
Article22. Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000; 7:203–14.
Article23. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7:335–6.
Article24. Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012; 7:e34233.
Article25. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006; 444:881–7.
Article26. Jones ML, Martoni CJ, Tamber S, Parent M, Prakash S. Evaluation of safety and tolerance of microencapsulated Lactobacillus reuteri NCIMB 30242 in a yogurt formulation: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol. 2012; 50:2216–23.
Article27. Chung HJ, Yu JG, Lee IA, Liu MJ, Shen YF, Sharma SP, et al. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio. 2016; 6:64–76.
Article28. Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients. 2019; 11:2704.
Article29. Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004; 53:1253–60.
Article30. Xie C, Halegoua-DeMarzio D. Role of probiotics in non-alcoholic fatty liver disease: does gut microbiota matter? Nutrients. 2019; 11:2837.
Article31. Mayes PA, Topping DL. Regulation of hepatic lipogenesis by plasma free fatty acids: simultaneous studies on lipoprotein secretion, cholesterol synthesis, ketogenesis and gluconeogenesis. Biochem J. 1974; 140:111–4.32. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPAREG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016; 39:1108–14.
Article33. Li H, Liu F, Lu J, Shi J, Guan J, Yan F, et al. Probiotic mixture of Lactobacillus plantarum strains improves lipid metabolism and gut microbiota structure in high fat diet-fed mice. Front Microbiol. 2020; 11:512.
Article34. Bernstein EL, Koutkia P, Ljungquist K, Breu J, Canavan B, Grinspoon S. Acute regulation of adiponectin by free fatty acids. Metabolism. 2004; 53:790–3.
Article35. Getty-Kaushik L, Song DH, Boylan MO, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity (Silver Spring). 2006; 14:1124–31.
Article36. Asmar M, Asmar A, Simonsen L, Gasbjerg LS, Sparre-Ulrich AH, Rosenkilde MM, et al. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes. 2017; 66:2363–71.
Article37. Parkin SM, Walker K, Ashby P, Robinson DS. Effects of glucose and insulin on the activation of lipoprotin lipase and on protein-synthesis in rat adipose tissue. Biochem J. 1980; 188:193–9.
Article38. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009; 457:480–4.
Article39. Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009; 4:e7125.
Article40. Stojanov S, Berlec A, Strukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020; 8:1715.
Article41. Kim MH, Yun KE, Kim J, Park E, Chang Y, Ryu S, et al. Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep. 2020; 10:19417.
Article42. Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020; 12:1474.
Article43. Hu HJ, Park SG, Jang HB, Choi MK, Park KH, Kang JH, et al. Obesity alters the microbial community profile in Korean adolescents. PLoS One. 2015; 10:e0134333.
Article44. Thingholm LB, Ruhlemann MC, Koch M, Fuqua B, Laucke G, Boehm R, et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe. 2019; 26:252–64.
Article45. Gao H, Wen JJ, Hu JL, Nie QX, Chen HH, Xiong T, et al. Fermented Momordica charantia L. juice modulates hyperglycemia, lipid profile, and gut microbiota in type 2 diabetic rats. Food Res Int. 2019; 121:367–78.
Article46. Lee GH, Kumar S, Lee JH, Chang DH, Kim DS, Choi SH, et al. Genome sequence of Oscillibacter ruminantium strain GH1, isolated from rumen of Korean native cattle. J Bacteriol. 2012; 194:6362.
Article47. Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009; 58:1509–17.
Article48. Olivares M, Neyrinck AM, Potgens SA, Beaumont M, Salazar N, Cani PD, et al. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018; 61:1838–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Probiotic Therapy on Atopic Dermatitis in Children: A Randomized, Double-blind, Placebo-controlled Trial
- The Effect of Foeniculum Vulgare (Fennel) on Body Composition in Postmenopausal Women with Excess Weight: A Double-blind Randomized Placebo-controlled Trial
- Effect of Lactobacillus sakei, a Probiotic Derived from Kimchi, on Body Fat in Koreans with Obesity: A Randomized Controlled Study
- Onion peel extract reduces the percentage of body fat in overweight and obese subjects: a 12-week, randomized, double-blind, placebo-controlled study
- Effect of Lactobacillus gasseri BNR17 on Overweight and Obese Adults: A Randomized, Double-Blind Clinical Trial