Korean Circ J.
2023 Jan;53(1):34-46. 10.4070/kcj.2022.0210.
Development of a Zebrafish Larvae Model for Diabetic Heart Failure With Reduced Ejection Fraction
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2538103
- DOI: http://doi.org/10.4070/kcj.2022.0210
Abstract
- Background and Objectives
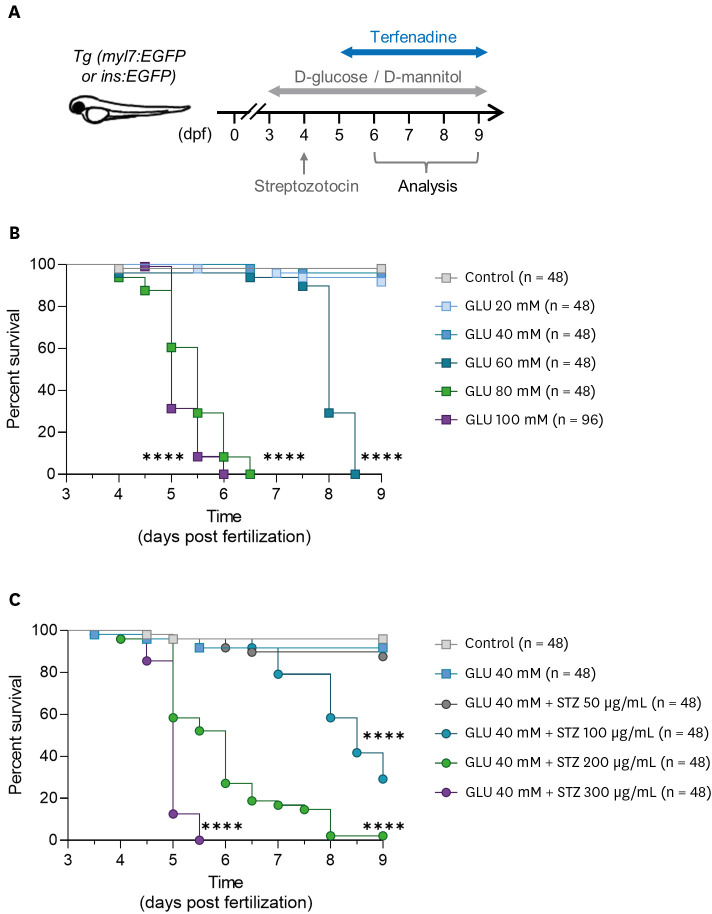
Diabetes mellitus (DM)-associated heart failure (HF) causes high morbidity and mortality. In this study, we established a zebrafish larvae model for in vivo research on diabetic HF.
Methods
DM-like phenotypes were induced by treating zebrafish larvae with a combination of D-glucose (GLU) and streptozotocin (STZ). HF was induced by treatment with terfenadine (TER), a potassium channel blocker. Additionally, myocardial contractility, motility, and viability were evaluated.
Results
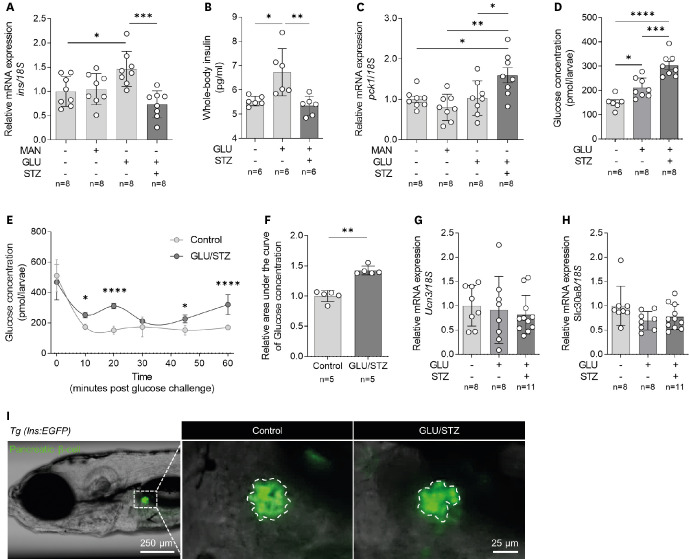
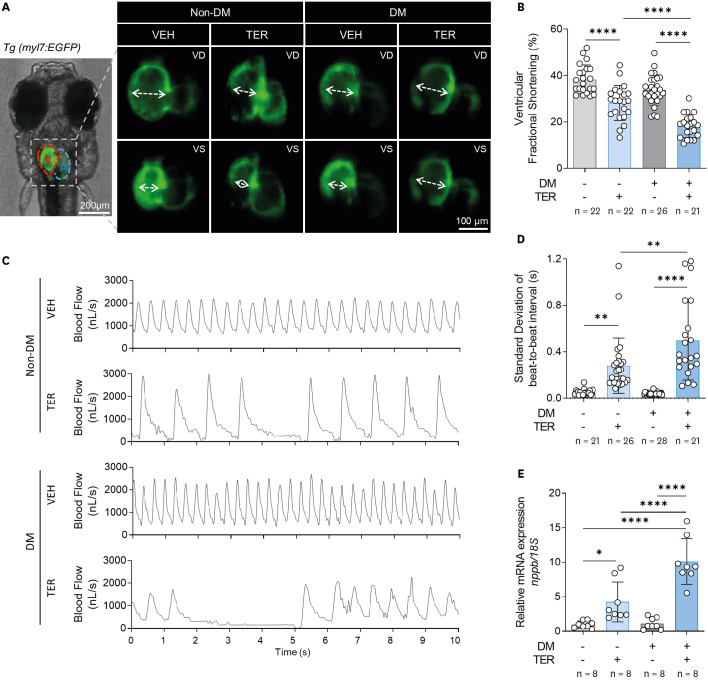
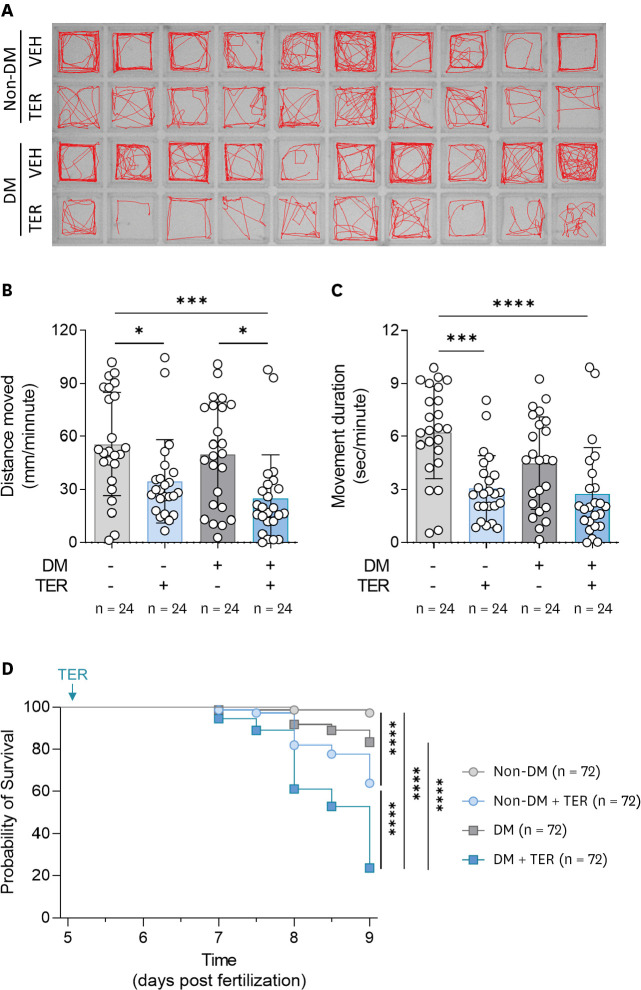
The zebrafish larvae treated with a combination of GLU and STZ showed significantly higher whole-body glucose concentrations, lower insulin levels, and higher phosphoenolpyruvate carboxykinase levels, which are markers of abnormal glucose homeostasis, than the group treated with only GLU, with no effect on viability. When treated with TER, DM zebrafish showed significantly less myocardial fractional shortening and more irregular contractions than the non-DM zebrafish. Furthermore, in DM-HF with reduced ejection fraction (rEF) zebrafish, a significant increase in the levels of natriuretic peptide B, a HF biomarker, markedly reduced motility, and reduced survival rates were observed.
Conclusions
We established a DM-HFrEF zebrafish model by sequentially treating zebrafish larvae with GLU, STZ, and TER. Our findings indicate the potential utility of the developed zebrafish larvae model not only in screening studies of new drug candidates for DM-HFrEF but also in mechanistic studies to understand the pathophysiology of DM-HFrEF.
Keyword
Figure
Cited by 1 articles
-
A Small Animal Model of Diabetic Heart Failure With Reduced Ejection Fraction
Seung-Hyun Jung, Hyun-Taek Kim
Korean Circ J. 2023;53(1):47-48. doi: 10.4070/kcj.2022.0349.
Reference
-
1. McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014; 2:843–851. PMID: 24731668.
Article2. Kong MG, Jang SY, Jang J, et al. Impact of diabetes mellitus on mortality in patients with acute heart failure: a prospective cohort study. Cardiovasc Diabetol. 2020; 19:49. PMID: 32359358.
Article3. Jang SY, Jang J, Yang DH, et al. Impact of insulin therapy on the mortality of acute heart failure patients with diabetes mellitus. Cardiovasc Diabetol. 2021; 20:180. PMID: 34496864.
Article4. Sharma A, Pagidipati NJ, Califf RM, et al. Impact of regulatory guidance on evaluating cardiovascular risk of new glucose-lowering therapies to treat type 2 diabetes mellitus: lessons learned and future directions. Circulation. 2020; 141:843–862. PMID: 31992065.
Article5. Parng C, Seng WL, Semino C, McGrath P. Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol. 2002; 1:41–48. PMID: 15090155.6. Grunwald DJ, Streisinger G. Induction of recessive lethal and specific locus mutations in the zebrafish with ethyl nitrosourea. Genet Res. 1992; 59:103–116. PMID: 1628817.
Article7. Jurczyk A, Roy N, Bajwa R, et al. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen Comp Endocrinol. 2011; 170:334–345. PMID: 20965191.8. Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013; 496:498–503. PMID: 23594743.9. Missinato MA, Zuppo DA, Watkins SC, Bruchez MP, Tsang M. Zebrafish heart regenerates after chemoptogenetic cardiomyocyte depletion. Dev Dyn. 2021; 250:986–1000. PMID: 33501711.10. Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003; 228:30–40. PMID: 12950077.
Article11. Quan H, Oh GC, Seok SH, Lee HY. Fimasartan, an angiotensin II receptor antagonist, ameliorates an in vivo zebrafish model of heart failure. Korean J Intern Med. 2020; 35:1400–1410. PMID: 32164398.
Article12. Gu G, Na Y, Chung H, Seok SH, Lee HY. Zebrafish Larvae Model of Dilated Cardiomyopathy Induced by Terfenadine. Korean Circ J. 2017; 47:960–969. PMID: 29035434.13. Members of the Panel on Euthanasia. AVMA guidelines for the euthanasia of animals: 2020 edition. Schaumburg (IL): American Veterinary Medical Association;2020.14. diIorio PJ, Moss JB, Sbrogna JL, Karlstrom RO, Moss LG. Sonic hedgehog is required early in pancreatic islet development. Dev Biol. 2002; 244:75–84. PMID: 11900460.15. Andersson O, Adams BA, Yoo D, et al. Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab. 2012; 15:885–894. PMID: 22608007.16. Lee Y, Yang J. Development of a zebrafish screening model for diabetic retinopathy induced by hyperglycemia: Reproducibility verification in animal model. Biomed Pharmacother. 2021; 135:111201. PMID: 33421732.17. Singh A, Castillo HA, Brown J, Kaslin J, Dwyer KM, Gibert Y. High glucose levels affect retinal patterning during zebrafish embryogenesis. Sci Rep. 2019; 9:4121. PMID: 30858575.18. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001; 50:537–546. PMID: 11829314.19. Huang CC, Chen PC, Huang CW, Yu J. Aristolochic acid induces heart failure in zebrafish embryos that is mediated by inflammation. Toxicol Sci. 2007; 100:486–494. PMID: 17823451.20. Matrone G, Taylor JM, Wilson KS, et al. Laser-targeted ablation of the zebrafish embryonic ventricle: a novel model of cardiac injury and repair. Int J Cardiol. 2013; 168:3913–3919. PMID: 23871347.
Article21. Jiménez-Amilburu V, Jong-Raadsen S, Bakkers J, Spaink HP, Marín-Juez R. GLUT12 deficiency during early development results in heart failure and a diabetic phenotype in zebrafish. J Endocrinol. 2015; 224:1–15. PMID: 25326603.
Article22. Kronlage M, Dewenter M, Grosso J, et al. O-GlcNAcylation of histone deacetylase 4 protects the diabetic heart from failure. Circulation. 2019; 140:580–594. PMID: 31195810.23. Jeon YW, Kim HC. Factors associated with awareness, treatment, and control rate of hypertension among Korean young adults aged 30-49 years. Korean Circ J. 2020; 50:1077–1091. PMID: 32975054.
Article24. Zang L, Shimada Y, Nishimura N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci Rep. 2017; 7:1461. PMID: 28469250.
Article25. Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008; 2008:704045. PMID: 19132099.
Article26. Tanaka S, Hayashi T, Toyoda T, et al. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metabolism. 2007; 56:1719–1728. PMID: 17998027.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal Management of Heart Failure with Preserve Ejection Fraction
- Prognostic Implications of Changes in Left Ventricular Ejection Fraction and Pulmonary Hypertension in Patients with Heart Failure with Reduced Ejection Fraction
- Total intravenous anesthesia using remimazolam for patients with heart failure with reduced ejection fraction: a case series
- Simple and Practical Way of Assessing Diastolic Function: Diastolic Heart Failure Revisited
- Zebrafish Larvae Model of Dilated Cardiomyopathy Induced by Terfenadine