Cancer Res Treat.
2023 Jan;55(1):304-313. 10.4143/crt.2022.004.
Busulfan, Melphalan, and Etoposide (BuME) Showed an Equivalent Effect to Busulfan, Cyclophosphamide, and Etoposide (BuCE) as Conditioning Therapy for Autologous Stem Cell Transplantation in Patients with Relapsed or High-Risk Non-Hodgkin’s Lymphoma: A Multicenter Randomized Phase II Study bythe Consortium for Improving Survival of Lymphoma (CISL)
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea
- 2Deparment of Internal Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 3Department of Internal Medicine, Konkuk University Medical Center and School of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Institute of Health Sciences, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea
- 5Division of Hematology-Oncology, Department of Medicine, Dongsan Medical Center, Keimyung University, Daegu, Korea
- 6Division of Hematology/Oncology, Department of Internal Medicine, Korea University School of Medicine, Seoul, Korea
- 7Department of Internal Medicine, Dong-A University College of Medicine, Pusan, Korea
- 8Division of Hematology-Oncology, Department of Internal Medicine, Pusan National University School of Medicine and Medical Research Institute, Pusan National University Hospital, Pusan, Korea
- 9Division of Hematology and Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 10Division of Hematology and Oncology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon, Korea
- 11Division of Oncology and Hematology, Department of Internal Medicine, Wonju Severance Christian Hospital,Yonsei University Wonju College of Medicine, Wonju, Korea
- 12Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea
- 13Department of Hematology/Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 14Division of Hematology/Oncology, Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea
- 15Division of Hematology-Oncology, Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Science, Seoul, Korea
- 16Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea
- KMID: 2538013
- DOI: http://doi.org/10.4143/crt.2022.004
Abstract
- Purpose
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is the standard management for relapsed or high-risk non-Hodgkin’s lymphoma (NHL). We reported the busulfan, melphalan, and etoposide (BuME) conditioning regimen was effective in patients with relapsed or high-risk NHL. Moreover, the busulfan, cyclophosphamide, and etoposide (BuCE) conditioning regimen has been used widely in ASCT for NHL. Therefore, based on these encouraging results, this randomized phase II multicenter trial compared the outcomes of BuME and BuCE as conditioning therapies for ASCT in patients with NHL.
Materials and Methods
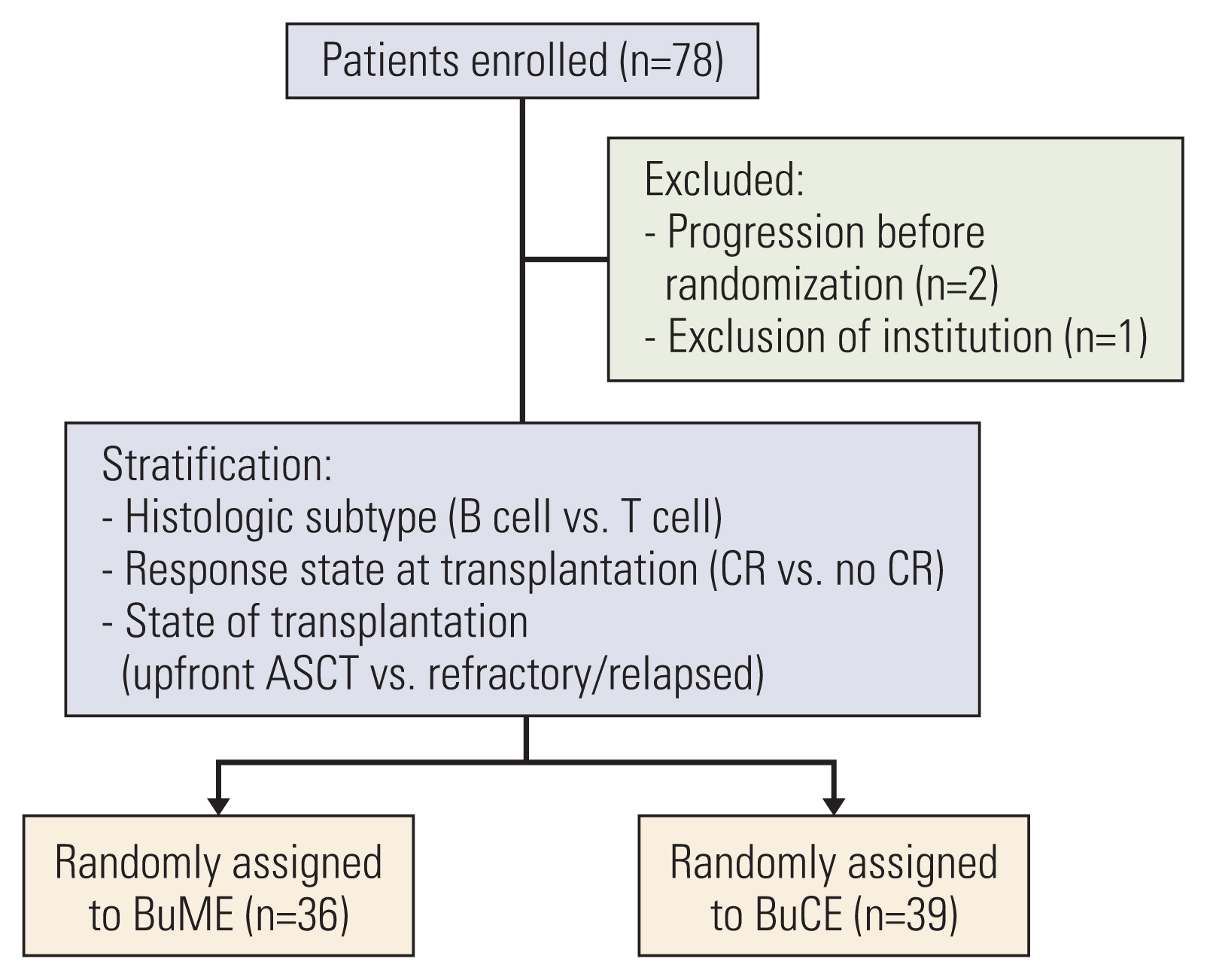
Patients were randomly assigned to receive either BuME (n=36) or BuCE (n=39). The BuME regimen was comprised of busulfan (3.2 mg/kg/day, intravenously) administered on days –7, –6, and –5, etoposide (400 mg/m2 intravenously) on days –5 and –4, and melphalan (50 mg/m2/day intravenously) on days –3 and –2. The BuCE regimen was comprised of busulfan (3.2 mg/kg/day intravenously) on days –7, –6, and –5, etoposide (400 mg/m2/day intravenously) on days –5 and –4, and cyclophosphamide (50 mg/kg/day intravenously) on days –3 and –2. The primary endpoint was 2-year progression-free survival (PFS).
Results
Seventy-five patients were enrolled. Eleven patients (30.5%) in the BuME group and 13 patients (33.3%) in the BuCE group had disease progression or died. The 2-year PFS rate was 65.4% in the BuME group and 60.6% in the BuCE group (p=0.746). There were no non-relapse mortalities within 100 days after transplantation.
Conclusion
There were no significant differences in PFS between the two groups. Therefore, busulfan-based conditioning regimens, BuME and BuCE, may be important treatment substitutes for the BCNU-containing regimens.
Figure
Reference
-
References
1. Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995; 333:1540–5.2. Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1995; 13:588–95.3. Salar A, Sierra J, Gandarillas M, Caballero MD, Marin J, Lahuerta JJ, et al. Autologous stem cell transplantation for clinically aggressive non-Hodgkin’s lymphoma: the role of preparative regimens. Bone Marrow Transplant. 2001; 27:405–12.4. Philip T, Chauvin F, Armitage J, Bron D, Hagenbeek A, Biron P, et al. Parma international protocol: pilot study of DHAP followed by involved-field radiotherapy and BEAC with autologous bone marrow transplantation. Blood. 1991; 77:1587–92.5. Kim JG, Sohn SK, Chae YS, Yang DH, Lee JJ, Kim HJ, et al. Multicenter study of intravenous busulfan, cyclophosphamide, and etoposide (i.v. Bu/Cy/E) as conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2007; 40:919–24.6. Isidori A, Christofides A, Visani G. Novel regimens prior to autologous stem cell transplantation for the management of adults with relapsed/refractory non-Hodgkin lymphoma and Hodgkin lymphoma: alternatives to BEAM conditioning. Leuk Lymphoma. 2016; 57:2499–509.
Article7. Kuittinen T, Husso-Saastamoinen M, Sipola P, Vuolteenaho O, Ala-Kopsala M, Nousiainen T, et al. Very acute cardiac toxicity during BEAC chemotherapy in non-Hodgkin’s lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2005; 36:1077–82.8. Kuittinen T, Jantunen E, Vanninen E, Mussalo H, Vuolteenaho O, Ala-Kopsala M, et al. Cardiac effects within 3 months of BEAC high-dose therapy in non-Hodgkin’s lymphoma patients undergoing autologous stem cell transplantation. Eur J Haematol. 2006; 77:120–7.9. Lemieux C, Ahmad I, Bambace NM, Bernard L, Cohen S, Delisle JS, et al. Outcome of autologous hematopoietic stem cell transplant in older patients with B cell lymphoma when selected for fitness and chemosensitive disease. Leuk Res. 2019; 79:75–80.10. Kim KH, Kim WS, Kim SJ, Yoon DH, Suh C, Kang HJ, et al. Treatment with intravenous busulfan, melphalan, and etoposide followed by autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma: a multicenter study from the consortium for improving survival of lymphoma. Transpl Int. 2020; 33:1211–9.11. Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988; 6:1562–8.12. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999; 17:1244.13. Robinson SP, Boumendil A, Finel H, Dreger P, Sureda A, Hermine O, et al. High-dose therapy with BEAC conditioning compared to BEAM conditioning prior to autologous stem cell transplantation for non-Hodgkin lymphoma: no differences in toxicity or outcome. A matched-control study of the EBMT-Lymphoma Working Party. Bone Marrow Transplant. 2018; 53:1553–9.14. Redondo AM, Valcarcel D, Gonzalez-Rodriguez AP, Suarez-Lledo M, Bello JL, Canales M, et al. Bendamustine as part of conditioning of autologous stem cell transplantation in patients with aggressive lymphoma: a phase 2 study from the GELTAMO group. Br J Haematol. 2019; 184:797–807.15. Lee SC, Kim SJ, Lee DH, Kim WS, Suh C, Won JH. Excessive toxicity of once daily i.v. BU, melphalan and thiotepa followed by auto SCT on patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2010; 45:801–2.16. Avalos BR, Klein JL, Kapoor N, Tutschka PJ, Klein JP, Copelan EA. Preparation for marrow transplantation in Hodgkin’s and non-Hodgkin’s lymphoma using Bu/CY. Bone Marrow Transplant. 1993; 12:133–8.17. Dalle JH, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Transplant. 2016; 22:400–9.18. Copelan EA, Penza SL, Pohlman B, Avalos BR, Goormastic M, Andresen SW, et al. Autotransplantation following busulfan, etoposide and cyclophosphamide in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2000; 25:1243–8.19. Yanamandra U, Gupta S, Khadwal A, Malhotra P. Melphalan-induced cardiotoxicity: ventricular arrhythmias. BMJ Case Rep. 2016; 2016:bcr2016218652.20. Jagadeesh D, Majhail NS, He Y, Ahn KW, Litovich C, Ahmed S, et al. Outcomes of rituximab-BEAM versus BEAM conditioning regimen in patients with diffuse large B cell lymphoma undergoing autologous transplantation. Cancer. 2020; 126:2279–87.21. Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol. 2013; 31:1662–8.22. Jo JC, Kim JS, Lee JH, Lee JH, Im SN, Lee SM, et al. Busulfan, etoposide, cytarabine, and melphalan as a high-dose regimen for autologous stem cell transplantation in peripheral T-cell lymphomas. Ann Hematol. 2021; 100:189–96.23. Park SI, Horwitz SM, Foss FM, Pinter-Brown LC, Carson KR, Rosen ST, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019; 125:1507–17.24. Roerden M, Walz JS, Muller MR, Sokler M, Federmann B, Kanz L, et al. The role of autologous stem cell transplantation in peripheral T cell lymphoma: a long-term follow-up single-center experience. J Cancer Res Clin Oncol. 2019; 145:2595–604.25. Zahid U, Akbar F, Amaraneni A, Husnain M, Chan O, Riaz IB, et al. A review of autologous stem cell transplantation in lymphoma. Curr Hematol Malig Rep. 2017; 12:217–26.26. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019; 380:45–56.27. Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016; 127:1410–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Yttrium-90 ibritumomab tiuxetan plus busulfan, cyclophosphamide, and etoposide (BuCyE) versus BuCyE alone as a conditioning regimen for non-Hodgkin lymphoma
- Safety and efficacy of bendamustine in the conditioning regimen for autologous stem cell transplantation in patients with relapsed/refractory lymphoma
- A Preliminary Report of Busulfan, Melphalan and Thiotepa or TBI-containing Bi-alkylator Chemotherapy as a Preparative Regimen for Allogeneic Bone Marrow Transplantation in Refractory or Relapsed Acute Leukemias
- High Dose Chemotherapy Using CD34+ Hematopoietic Stem Cells
- Patient profiles and outcomes of lymphoma patients who underwent autologous stem cell transplant in National Kidney and Transplant Institute: a single-center analysis