Blood Res.
2019 Jun;54(2):108-113. 10.5045/br.2019.54.2.108.
Safety and efficacy of bendamustine in the conditioning regimen for autologous stem cell transplantation in patients with relapsed/refractory lymphoma
- Affiliations
-
- 1Hematology/Oncology and Bone Marrow Transplantation, Aga Khan University Hospital, Karachi, Pakistan. munira.moosajee@aku.edu
- 2Department of Oncology, Aga Khan University Hospital, Karachi, Pakistan.
- 3Department of Community Health Sciences, Aga Khan University Hospital, Karachi, Pakistan.
- 4Hematology and Laboratory Medicine, Aga Khan University Hospital, Karachi, Pakistan.
- KMID: 2451010
- DOI: http://doi.org/10.5045/br.2019.54.2.108
Abstract
- BACKGROUND
Bendamustine is an attractive option for the management of both de novo and relapsed lymphomas. It is being increasingly used in the conditioning regimen for autologous stem cell transplantation (SCT) and can be an alternative to the traditionally-used carmustine. In this study, we aimed to determine the safety and efficacy of bendamustine in the conditioning regimen for autologous SCT in refractory/relapsed lymphomas.
METHODS
We designed a descriptive study to evaluate bendamustine in combination with etoposide, cytarabine, and melphalan (BeEAM) in the conditioning regimen for autologous SCT.
RESULTS
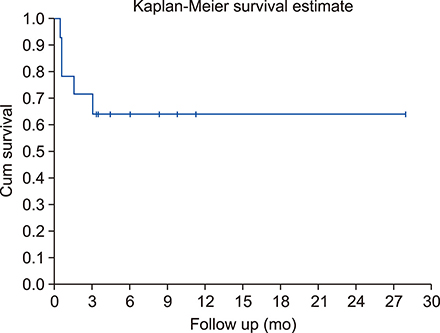
Fourteen patients (median age, 28 yr) with Hodgkin's lymphoma (HL) (N=8), non-Hodgkin's lymphomas (NHL) (N=5), or peripheral T-cell lymphoma, not otherwise specified (PTCL NOS) (N=1) were included in the study. A median number of 5.95×10ⶠCD34+ cells/kg were transfused. Median times to absolute neutrophil count and platelet engraftment were 17 days and 24 days, respectively. The 100-day transplantation mortality rate was 28% (4 patients). Eight patients (57.14%) had GII-III acute kidney injury, four patients (28.5%) had GIII-IV hyperbilirubinemia, and twelve patients (85%) had GII-III diarrhea. After 3 months, 37% (5 patients) and 21.4% (3 patients) demonstrated complete response and partial response, respectively. The median follow-up was 5.5 months (15 days-19 mo). At the final follow-up, 7 patients (50%) were alive and in CR.
CONCLUSION
Our study showed that bendamustine is a potentially toxic agent in the conditioning regimen for autologous SCT, resulting in significant liver, kidney, and gastrointestinal toxicity. Further studies are required to assess its safety and efficacy at reduced doses.
MeSH Terms
-
Acute Kidney Injury
Bendamustine Hydrochloride*
Blood Platelets
Carmustine
Cytarabine
Diarrhea
Etoposide
Follow-Up Studies
Hodgkin Disease
Humans
Hyperbilirubinemia
Kidney
Liver
Lymphoma*
Lymphoma, Non-Hodgkin
Lymphoma, T-Cell, Peripheral
Melphalan
Mortality
Neutrophils
Stem Cell Transplantation*
Stem Cells*
Bendamustine Hydrochloride
Carmustine
Cytarabine
Etoposide
Melphalan
Figure
Reference
-
1. Kuruvilla J. Standard therapy of advanced Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009; 2009:497–506.
Article2. Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004; 103:3684–3688.
Article3. Ardeshna KM, Kakouros N, Qian W, et al. Conventional second-line salvage chemotherapy regimens are not warranted in patients with malignant lymphomas who have progressive disease after first-line salvage therapy regimens. Br J Haematol. 2005; 130:363–372.
Article4. Bierman PJ, Armitage JO. Looking back (and ahead) at salvage treatment for non-Hodgkin lymphoma. Oncology (Williston Park). 2009; 23:619.5. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995; 333:1540–1545.
Article6. Reddy NM, Oluwole O, Greer JP, Engelhardt BG, Jagasia MH, Savani BN. Outcomes of autologous or allogeneic stem cell transplantation for non-Hodgkin lymphoma. Exp Hematol. 2014; 42:39–45.
Article7. Akhtar S. High dose chemotherapy and autologous stem cell transplantation in relapsed or refractory Hodgkin lymphoma: Emerging questions, newer agents, and changing paradigm. Hematol Oncol Stem Cell Ther. 2017; 10:272–276.
Article8. Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 1995; 13:588–595.
Article9. Puig N, de la Rubia J, Remigia MJ, et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma. 2006; 47:1488–1494.
Article10. Liu HW, Seftel MD, Rubinger M, et al. Total body irradiation compared with BEAM: Long-term outcomes of peripheral blood autologous stem cell transplantation for non-Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 2010; 78:513–520.
Article11. Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008; 14:309–317.
Article12. Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin's lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol. 2008; 26:204–210.
Article13. Brugger W, Ghielmini M. Bendamustine in indolent non-Hodgkin's lymphoma: a practice guide for patient management. Oncologist. 2013; 18:954–964.
Article14. Visani G, Malerba L, Stefani PM, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011; 118:3419–3425.
Article15. Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28:4184–4190.
Article16. Caimi PF, William BM, Silva Rondon CH, et al. Comparison of 2 carmustine-containing regimens in the rituximab era: excellent outcomes even in poor-risk patients. Biol Blood Marrow Transplant. 2015; 21:1926–1931.
Article17. Czyz A, Lojko-Dankowska A, Dytfeld D, et al. Prognostic factors and long-term outcome of autologous haematopoietic stem cell transplantation following a uniform-modified BEAM-conditioning regimen for patients with refractory or relapsed Hodgkin lymphoma: a single-center experience. Med Oncol. 2013; 30:611.
Article18. Reid RM, Baran A, Friedberg JW, et al. Outpatient administration of BEAM conditioning prior to autologous stem cell transplantation for lymphoma is safe, feasible, and cost-effective. Cancer Med. 2016; 5:3059–3067.
Article19. Galieni P, Troiani E, Bigazzi C, et al. Modified BEAM as conditioning regimen for lymphoma patients undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018; 53:91–93.
Article20. van Besien K, Tabocoff J, Rodriguez M, et al. High-dose chemotherapy with BEAC regimen and autologous bone marrow transplantation for intermediate grade and immunoblastic lymphoma: durable complete remissions, but a high rate of regimen-related toxicity. Bone Marrow Transplant. 1995; 15:549–555.21. Gribben JG, Linch DC, Singer CR, McMillan AK, Jarrett M, Goldstone AH. Successful treatment of refractory Hodgkin's disease by high-dose combination chemotherapy and autologous bone marrow transplantation. Blood. 1989; 73:340–344.
Article22. Visani G, Picardi P, Tosi P, et al. Autologous stem cell transplantation for aggressive lymphomas. Mediterr J Hematol Infect Dis. 2012; 4:e2012075.
Article23. Sun CL, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood. 2011; 118:4723–4731.
Article24. Chantepie S, Tchernonog E, Peyrade F, et al. Bendamustine-based (BeEAM) conditioning before autologous stem cell transplantation: result of a French multicenter study of 386 patients from Lysa Centers. Blood (ASH Annual Meeting Abstracts). 2016; 128:Suppl. abst 3450.
Article25. Garciaz S, Coso D, Schiano de Collela JM, et al. Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: an increasing risk of renal toxicity. Bone Marrow Transplant. 2016; 51:319–321.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Patient profiles and outcomes of lymphoma patients who underwent autologous stem cell transplant in National Kidney and Transplant Institute: a single-center analysis
- Bendamustine in combination with ifosfamide, etoposide, and vinorelbine (VIBE) is an effective salvage regimen for heavily pre-treated patients with relapsed or refractory Hodgkin lymphoma: a single-center experience
- Treatment of Relapsed Hodgkin Lymphoma
- Yttrium-90 ibritumomab tiuxetan plus busulfan, cyclophosphamide, and etoposide (BuCyE) versus BuCyE alone as a conditioning regimen for non-Hodgkin lymphoma
- BEAM conditioning regimen and autologous peripheral stem cell transplantation in patients with malignant lymphoma