Blood Res.
2022 Dec;57(4):264-271. 10.5045/br.2022.2022194.
Reduced-intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for patients with myelofibrosis
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2537549
- DOI: http://doi.org/10.5045/br.2022.2022194
Abstract
- Background
Allogeneic hematopoietic stem cell transplantation (alloSCT) is the sole curative option for myelofibrosis (MF). However, it is unknown as to which of the two, myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC), is a better preconditioning regimen.
Methods
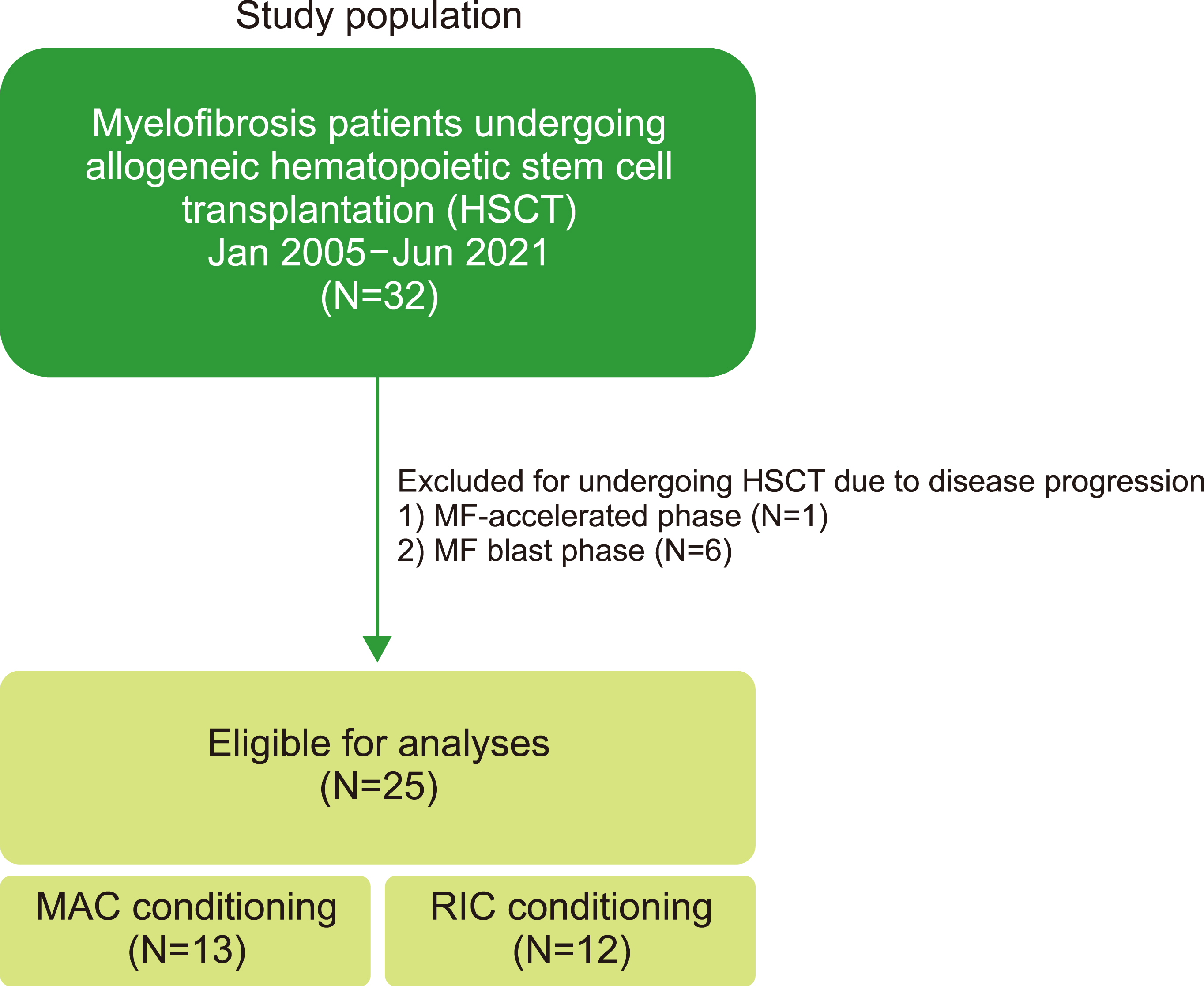
Twenty-five patients with MF were treated with alloSCT, 12 of whom underwent RIC. Baseline characteristics, response to alloSCT, adverse events, including graft-versus-host disease (GVHD), and survival outcomes were reviewed.
Results
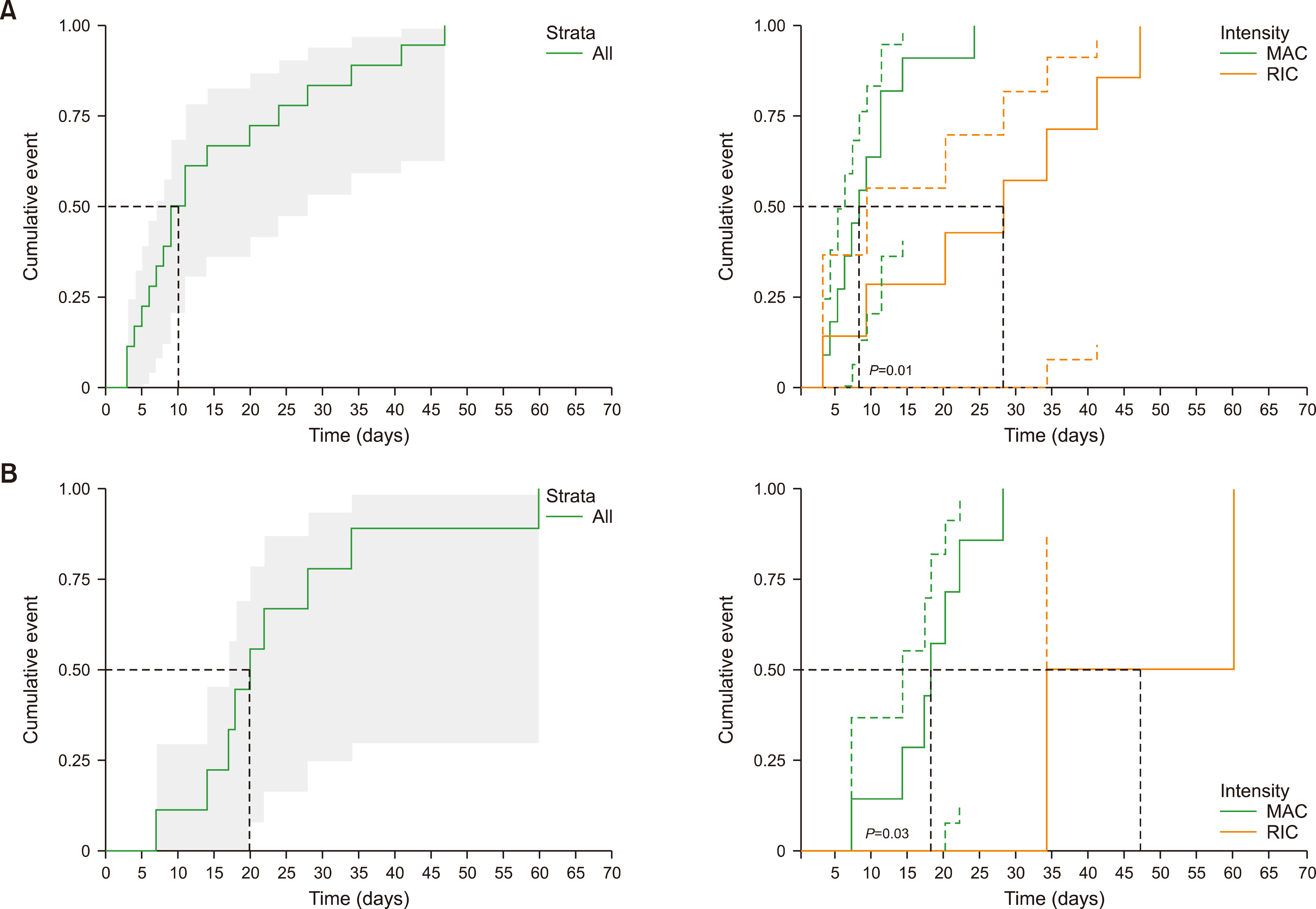
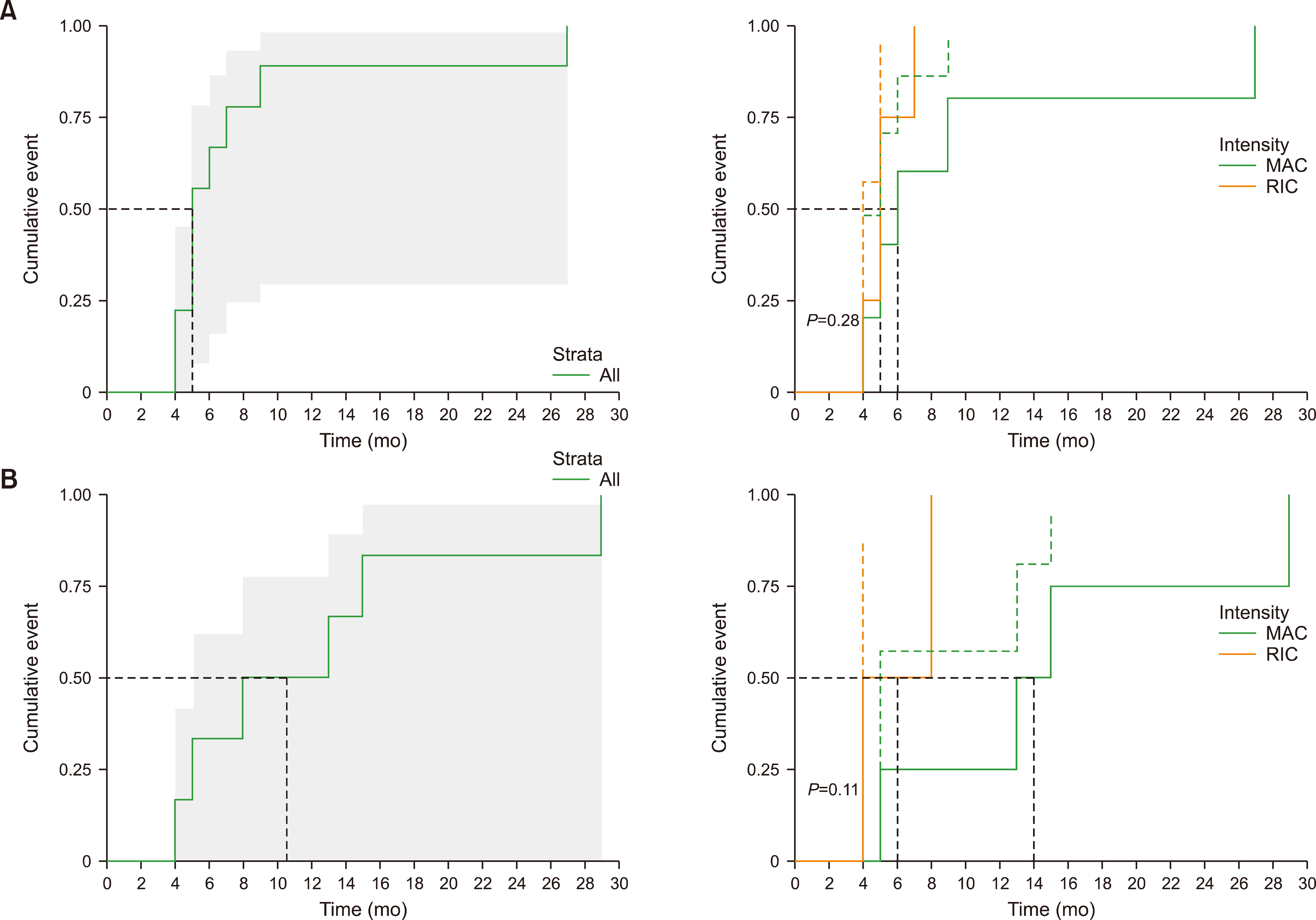
There was no difference in the neutrophil engraftment rate and time to engraftment between MAC vs. RIC. The time to platelet engraftment was significantly longer in the MAC group (median, 112.8 vs. 28.8 days for MAC vs. RIC, respectively, P =0.049). RIC was more advantageous in terms of achieving complete chimerism (38.5% vs. 83.3%, P =0.041). The incidence of acute GVHD was 84.6% (11 of 13) and 58.3% (7 of 12) in the MAC and RIC groups, respectively. The cumulative incidence of grade III‒IV acute GVHD was significantly higher in the MAC group than in the RIC group (P =0.03). No significant differences were observed in progression-free and overall survival. The 17-month probability of progression-free survival was 38.4% [95% confidence interval (CI), 19.3‒76.5] vs. 47.6% (95% CI, 25.7‒88.2) (P =0.21), and that of overall survival was 53.8% (95% CI, 32.5‒89.1) vs. 48.6% (95% CI, 26.8‒88.3) (P =0.85) for MAC vs. RIC, respectively.
Conclusion
RIC offers a significant advantage over MAC, even in younger patients with MF undergoing alloSCT, in terms of cell engraftment, rate of complete chimerism achievement, and incidence of acute GVHD.
Figure
Reference
-
1. Castro-Malaspina H, Moore MA. 1982; Pathophysiological mechanisms operating in the development of myelofibrosis: role of megakaryocytes. Nouv Rev Fr Hematol (1978). 24:221–6. PMID: 6292827.2. Wolf BC, Neiman RS. 1985; Myelofibrosis with myeloid metaplasia: pathophysiologic implications of the correlation between bone marrow changes and progression of splenomegaly. Blood. 65:803–9. DOI: 10.1182/blood.V65.4.803.803. PMID: 3978228.3. Arber DA, Orazi A, Hasserjian R, et al. 2016; The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127:2391–405. DOI: 10.1182/blood-2016-03-643544. PMID: 27069254.4. O'Sullivan JM, Harrison CN. 2018; Myelofibrosis: clinicopathologic features, prognosis, and management. Clin Adv Hematol Oncol. 16:121–31. PMID: 29741513.5. Kröger NM, Deeg JH, Olavarria E, et al. 2015; Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 29:2126–33. DOI: 10.1038/leu.2015.233. PMID: 26293647.6. Gupta V, Hari P, Hoffman R. 2012; Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 120:1367–79. DOI: 10.1182/blood-2012-05-399048. PMID: 22700718. PMCID: PMC5800543.7. Kerbauy DM, Gooley TA, Sale GE, et al. 2007; Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant. 13:355–65. DOI: 10.1016/j.bbmt.2006.11.004. PMID: 17317589.8. Guardiola P, Anderson JE, Bandini G, et al. 1999; Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European group for blood and marrow transplantation, Société Française de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood. 93:2831–8. PMID: 10216077.9. Byun JM, Kim YJ, Youk T, Yang JJ, Yoo J, Park TS. 2017; Real world epidemiology of myeloproliferative neoplasms: a population based study in Korea 2004-2013. Ann Hematol. 96:373–81. DOI: 10.1007/s00277-016-2902-9. PMID: 28028559.10. McLornan DP, Yakoub-Agha I, Robin M, Chalandon Y, Harrison CN, Kroger N. 2019; State-of-the-art review: allogeneic stem cell transplantation for myelofibrosis in 2019. Haematologica. 104:659–68. DOI: 10.3324/haematol.2018.206151. PMID: 30872371. PMCID: PMC6442950.11. Robin M, Porcher R, Wolschke C, et al. 2016; Outcome after transplantation according to reduced-intensity conditioning regimen in patients undergoing transplantation for myelofibrosis. Biol Blood Marrow Transplant. 22:1206–11. DOI: 10.1016/j.bbmt.2016.02.019. PMID: 26970380.12. McLornan D, Szydlo R, Koster L, et al. 2019; Myeloablative and reduced-intensity conditioned allogeneic hematopoietic stem cell transplantation in myelofibrosis: a retrospective study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 25:2167–71. DOI: 10.1016/j.bbmt.2019.06.034. PMID: 31284069.13. Glucksberg H, Storb R, Fefer A, et al. 1974; Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 18:295–304. DOI: 10.1097/00007890-197410000-00001. PMID: 4153799.14. Jagasia MH, Greinix HT, Arora M, et al. 2015; National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 21:389–401. e1. DOI: 10.1016/j.bbmt.2014.12.001. PMID: 25529383. PMCID: PMC4329079.15. Copelan E, Casper JT, Carter SL, et al. 2007; A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 13:1469–76. DOI: 10.1016/j.bbmt.2007.08.047. PMID: 18022577.16. Delhommeau F, Jeziorowska D, Marzac C, Casadevall N. 2010; Molecular aspects of myeloproliferative neoplasms. Int J Hematol. 91:165–73. DOI: 10.1007/s12185-010-0530-z. PMID: 20186505.17. Verstovsek S, Mesa RA, Gotlib J, et al. 2012; A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 366:799–807. DOI: 10.1056/NEJMoa1110557. PMID: 22375971. PMCID: PMC4822164.18. Harrison C, Kiladjian JJ, Al-Ali HK, et al. 2012; JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 366:787–98. DOI: 10.1056/NEJMoa1110556. PMID: 22375970.19. Mascarenhas J, Hoffman R. 2012; Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 18:3008–14. DOI: 10.1158/1078-0432.CCR-11-3145. PMID: 22474318.20. Ciurea SO, Sadegi B, Wilbur A, et al. 2008; Effects of extensive splenomegaly in patients with myelofibrosis undergoing a reduced intensity allogeneic stem cell transplantation. Br J Haematol. 141:80–3. DOI: 10.1111/j.1365-2141.2008.07010.x. PMID: 18324970.21. Polverelli N, Mauff K, Kröger N, et al. 2021; Impact of spleen size and splenectomy on outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis: a retrospective analysis by the Chronic Malignancies Working Party on behalf of European Society for Blood and Marrow Transplantation (EBMT). Am J Hematol. 96:69–79. DOI: 10.1002/ajh.26020. PMID: 33064301.22. Akpek G, Pasquini MC, Logan B, et al. 2013; Effects of spleen status on early outcomes after hematopoietic cell transplantation. Bone Marrow Transplant. 48:825–31. DOI: 10.1038/bmt.2012.249. PMID: 23222382. PMCID: PMC3606905.23. Jain T, Kunze KL, Temkit M, et al. 2019; Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 54:204–11. DOI: 10.1038/s41409-018-0226-1. PMID: 29795431. PMCID: PMC8201429.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allogeneic Stem Cell Transplantation for Patients with Advanced Hematological Malignancies: Comparison of Fludarabine-based Reduced Intensity Conditioning versus Myeloablative Conditioning
- Graft-versus-Leukemia Effect of Nonmyeloablative Stem Cell Transplantation
- Treosulfan-Based Conditioning Regimen for Hematopoietic Stem Cell Transplantation in Pediatric Patients with Hemophagocytic Lymphohistiocytosis
- Reduced Intensity Conditioning Regimen Followed by Allogeneic Peripheral Blood Stem Cell Transplantation for Acute Panmyelosis with Myelofibrosis
- Unrelated Bone Marrow Transplantation with a Reduced Toxicity Myeloablative Conditioning Regimen in Wiskott-Aldrich Syndrome