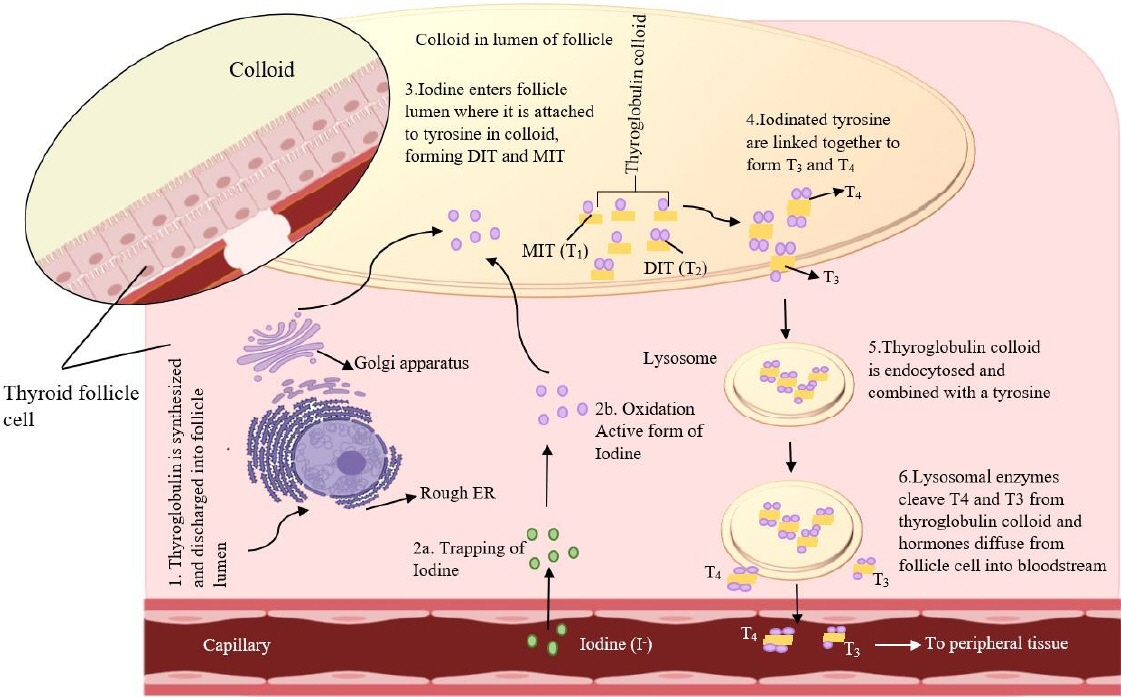
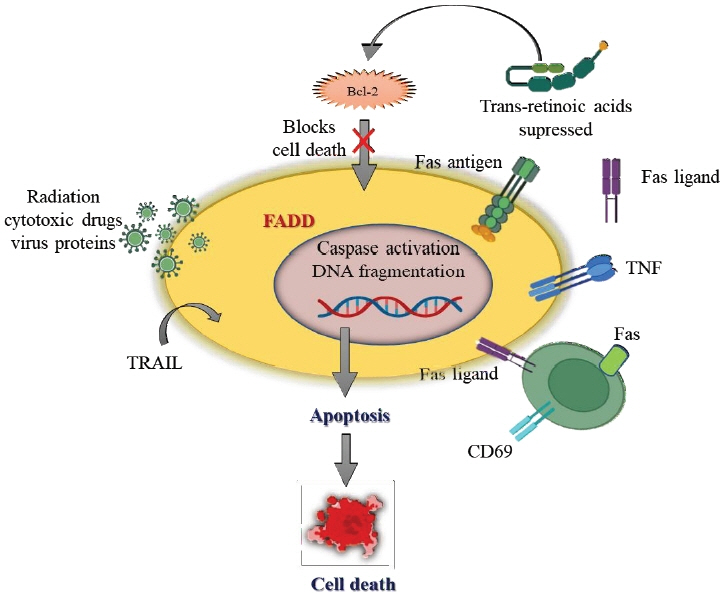
Ann Pediatr Endocrinol Metab.
2022 Dec;27(4):256-264. 10.6065/apem.2244186.093.
Impact of iodine intake on the pathogenesis of autoimmune thyroid disease in children and adults
- Affiliations
-
- 1Human Cytogenetics and Genomics Laboratory, Faculty of Allied Health Sciences, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Tamilnadu, India
- KMID: 2537237
- DOI: http://doi.org/10.6065/apem.2244186.093
Abstract
- Thyroid hormone (TH) regulates the body's metabolism and iodine, a vital trace mineral, is vital for TH synthesis. As a TH biosynthesis catalyst, iodine has a substantial role in our health. When there is a modest iodine deficit, the thyroid gland grows autonomously, resulting in thyrotoxicosis. Those who consume excessive iodine risk developing hypothyroidism and thyroid autoimmunity. A transient hyperthyroid condition may rapidly increase iodine consumption. Iodine deficiency is common across the globe, and provision of supplementary iodine, in forms such as iodized salt or vegetable oil, has many benefits. Vegetarians, for instance, may not consume adequate amounts of iodine in some countries with high iodine content. Reduced dietary iodine intakes may be a consequence of efforts to reduce salt intakes to prevent hypertension. In addition, iodine consumption is decreasing in many countries, even among those where endemic goiter has previously been eradicated, leading to the re-emergence of iodine-deficiency-related disorders such as goiter. This review will discuss how iodine can contribute to the development of thyroid disease.
Keyword
Figure
Reference
-
References
1. McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012; 42:252–65.2. Sengupta J, Das H, Vinoth GCD, Sasithra S, Jennifer BJ. An observational study of incidence of metabolic syndrome among patients with controlled Grave's disease. Clin Epidemiology Glob Health. 2022; 15:101010.3. Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol. 2014; 170:R241–52.4. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019; 78:34–44.5. Nacamulli D, Petricca D, Mian C. Selenium and autoimmune thyroiditis. J Endocrinol Invest. 2013; 36(10 Suppl):8–14.6. Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011; 364:1920–31.7. Hu S, Rayman MP. Multiple nutritional factors and the risk of Hashimoto's thyroiditis. Thyroid. 2017; 27:597–610.8. Rajan VK. Raised incidence of autoimmune thyroiditis among females in 2nd, 3rd and 4th decades: a randomized study. Inte Surg J. 2019; 6:1074–7.9. Marković L. Role of iodine in pathogenesis of thyroid disease-is induction of apoptosis consequence of iodine cytotoxicity? Srp Arh Celok Lek. 2017; 145:309–14.10. Fuge R, Johnson CC. Iodine and human health, the role of environmental geochemistry and diet, a review. Appl Geochemistry. 2015; 63:282–302.11. Chen W, Li X, Wu Y, Bian J, Shen J, Jiang W, et al. Associations between iodine intake, thyroid volume, and goiter rate in school-aged Chinese children from areas with high iodine drinking water concentrations. Am J Clin Nutr. 2017; 105:228–33.12. Ovadia YS, Gefel D, Aharoni D, Turkot S, Fytlovich S, Troen AM. Can desalinated seawater contribute to iodinedeficiency disorders? An observation and hypothesis. Public Health Nutr. 2016; 19:2808–17.13. Ershow AG, Skeaff SA, Merkel JM, Pehrsson PR. Development of databases on iodine in foods and dietary supplements. Nutrients. 2018; 10:100.14. Nerhus I, Wik Markhus M, Nilsen BM, Øyen J, Maage A, Ødegård ER, et al. Iodine content of six fish species, Norwegian dairy products and hen's egg. Food Nutr Res. 2018; May. 24. 62:https://doi.org/10.29219/fnr.v62.1291. [Epub].15. van der Reijden OL, Zimmermann MB, Galetti V. Iodine in dairy milk: Sources, concentrations and importance to human health. Best Pract Res Clin Endocrinol Metab. 2017; 31:385–95.16. Maehre HK, Malde MK, Eilertsen KE, Elvevoll EO. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J Sci Food Agric. 2014; 94:3281–90.17. Bouga M, Combet E. Emergence of seaweed and seaweedcontaining foods in the UK: focus on labeling, iodine content, toxicity and nutrition. Foods. 2015; 4:240–53.18. Bürgi H. Iodine excess. Best Pract Res Clin Endocrinol Metab. 2010; 24:107–15.19. Pearce EN, editor. Iodine deficiency disorders and their elimination. Cham (Switzerland): Springer International Publishing;;2017.20. Aakre I, Tveito Evensen L, Kjellevold M, Dahl L, Henjum S, Alexander J, et al. Iodine status and thyroid function in a group of seaweed consumers in Norway. Nutrients. 2020; 12:3483.21. Gorstein JL, Bagriansky J, Pearce EN, Kupka R, Zimmermann MB. Estimating the health and economic benefits of universal salt iodization programs to correct iodine deficiency disorders. Thyroid. 2020; 30:1802–9.22. Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci. 2019; 1446:44–65.23. Teti C, Panciroli M, Nazzari E, Pesce G, Mariotti S, Olivieri A, et al. Iodoprophylaxis and thyroid autoimmunity: an update. Immunol Res. 2021; 69:129–38.24. Ruggeri RM, CampennÌ A, Giuffrida G, Casciaro M, Barbalace MC, Hrelia S, et al. Oxidative stress as a key feature of autoimmune thyroiditis: an update. Minerva Endocrinol. 2020; 45:326–44.25. Segni M. Disorders of the thyroid gland in infancy, childhood and adolescence. Feingold KR, Anawalt B, Boyce A, editors. South Dartmouth (MA). MDText.com, Inc.: 2000. [update 2017 Mar18; cited 2017 Mar 18]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279032/.26. Thyroid. In: Kappy M, Geffner M, Allen D. Pediatric practice. New York: McGraw-Hill Education, 2010. p. 107-10.27. Sperling M. Pediatric endocrinology. 4th. Philadelphia (PA): Elsevier, 2014:186-92. Chapter 7. Disorders of the thyroid in the newborn 401 and infant.28. Zimmermann MB. Iodine deficiency. Endocr Rev. 2009; 30:376–408.29. Wapnir IL, van de Rijn M, Nowels K, Amenta PS, Walton K, Montgomery K, et al. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab. 2003; 88:1880–8.30. Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, et al. The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008; 586:3813–24.31. Kopp P, Pesce L, Solis-S JC. Pendred syndrome and iodide transport in the thyroid. Trends Endocrinol Metab. 2008; 19:260–8.32. Twyffels L, Strickaert A, Virreira M, Massart C, Van Sande J, Wauquier C, et al. Anoctamin-1/TMEM16A is the major apical iodide channel of the thyrocyte. Am J Physiol Cell Physiol. 2014; 307:C1102–12.33. Carvalho DP, Dupuy C. Thyroid hormone biosynthesis and release. Mol Cell Endocrinol. 2017; 458:6–15.34. Gnidehou S, Caillou B, Talbot M, Ohayon R, Kaniewski J, Noël-Hudson MS, et al. Iodotyrosine dehalogenase 1 (DEHAL1) is a transmembrane protein involved in the recycling of iodide close to the thyroglobulin iodination site. FASEB J. 2004; 18:1574–6.35. De la Vieja A, Santisteban P. Role of iodide metabolism in physiology and cancer. Endocr Relat Cancer. 2018; 25:R225–45.36. Weir GC, Jameson JL, De Groot LJ. Endocrinology adult and pediatric: diabetes mellitus and obesity E-book. 6th ed. Philadelphia (PA): Elsevier Health Sciences;;2013.37. Chung HR. Iodine and thyroid function. Ann Pediatr Endocrinol Metab. 2014; 19:8–12.38. Pedersen IB, Laurberg P, Knudsen N, Jørgensen T, Perrild H, Ovesen L, et al. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007; 92:3122–7.39. Aghini Lombardi F, Fiore E, Tonacchera M, Antonangeli L, Rago T, Frigeri M, et al. The effect of voluntary iodine prophylaxis in a small rural community: the Pescopagano survey 15 years later. J Clin Endocrinol Metab. 2013; 98:1031–9.40. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013; 79:145–51.41. Effraimidis G, Tijssen JG, Wiersinga WM. Alcohol consumption as a risk factor for autoimmune thyroid disease: a prospective study. Eur Thyroid J. 2012; 1:99–104.42. Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007; 92:1263–8.43. Wiersinga WM. Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol Metab (Seoul). 2016; 31:213–22.44. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010; 20:755–61.45. Lin JD. The role of apoptosis in autoimmune thyroid disorders and thyroid cancer. BMJ. 2001; 322:1525–7.46. Simmonds MJ, Gough SC. The search for the genetic contribution to autoimmune thyroid disease: the never ending story? Brief Funct Genomics. 2011; 10:77–90.47. Inaba H, Martin W, Ardito M, De Groot AS, De Groot LJ. The role of glutamic or aspartic acid in position four of the epitope binding motif and thyrotropin receptorextracellular domain epitope selection in Graves' disease. J Clin Endocrinol Metab. 2010; 95:2909–16.48. Hodge SE, Ban Y, Strug LJ, Greenberg DA, Davies TF, Concepcion ES, et al. Possible interaction between HLADRbeta1 and thyroglobulin variants in Graves' disease. Thyroid. 2006; 16:351–5.49. André S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J. Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009; 174:1575–87.50. Fournié GJ, Mas M, Cautain B, Savignac M, Subra JF, Pelletier L, et al. Induction of autoimmunity through bystander effects. Lessons from immunological disorders induced by heavy metals. J Autoimmun. 2001; 16:319–26.51. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015; 14:174–80.52. Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011; 43:897–901.53. Simmonds MJ. Evaluating the role of B cells in autoimmune disease: more than just initiators of disease? In: Advances in medicine and biology. Hauppauge (NY): Nova Science Publishers, 2011:151-76.54. Castanet M, Polak M. Spectrum of Human Foxe1/TTF2 Mutations. Horm Res Paediatr. 2010; 73:423–9.55. Knaus UG. Oxidants in physiological processes. In: Schmidt HHHW, Ghezzi P, Cuadrado A, editors. Reactive oxygen species. Handbook of Experimental Pharmacology, vol 264. Cham (Switzerland): Springer, 2020:27-47.56. Karbownik-Lewińska M, Kokoszko-Bilska A. Oxidative damage to macromolecules in the thyroid - experimental evidence. Thyroid Res. 2012; 5:25.57. Iwan P, Stepniak J, Karbownik-Lewinska M. Pro-oxidative effect of KIO3 and protective effect of melatonin in the thyroid-comparison to other tissues. Life (Basel). 2021; 11:592.58. Rynkowska A, Stępniak J, Karbownik-Lewińska M. Fenton reaction-induced oxidative damage to membrane lipids and protective effects of 17β-estradiol in porcine ovary and thyroid homogenates. Int J Environ Res Public Health. 2020; 17:6841.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Status of Iodine Intake and Comparison of Characteristics according to Iodine-sourced Food Intake Patterns of Chinese Adults: A Study Encompassing Three Regions with Different Iodine Nutritional Statuses
- Iodine and thyroid function
- Evaluation of Iodine Status among Korean Patients with Papillary Thyroid Cancer Using Dietary and Urinary Iodine
- Effects of Increased Iodine Intake on Thyroid Disorders
- An overview of the pathogenic mechanisms of autoimmune thyroid disorders