Korean J Gastroenterol.
2022 Dec;80(6):273-280. 10.4166/kjg.2022.099.
A Pilot Study of Peritumor Administration of 5-FU for Preventing Bleeding in Advanced Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University College of Medicine, Seoul, Korea
- 2Department of Medical Research Institute, Kangbuk Samsung Hospital, Sungkyunkwan University College of Medicine, Seoul, Korea
- KMID: 2537099
- DOI: http://doi.org/10.4166/kjg.2022.099
Abstract
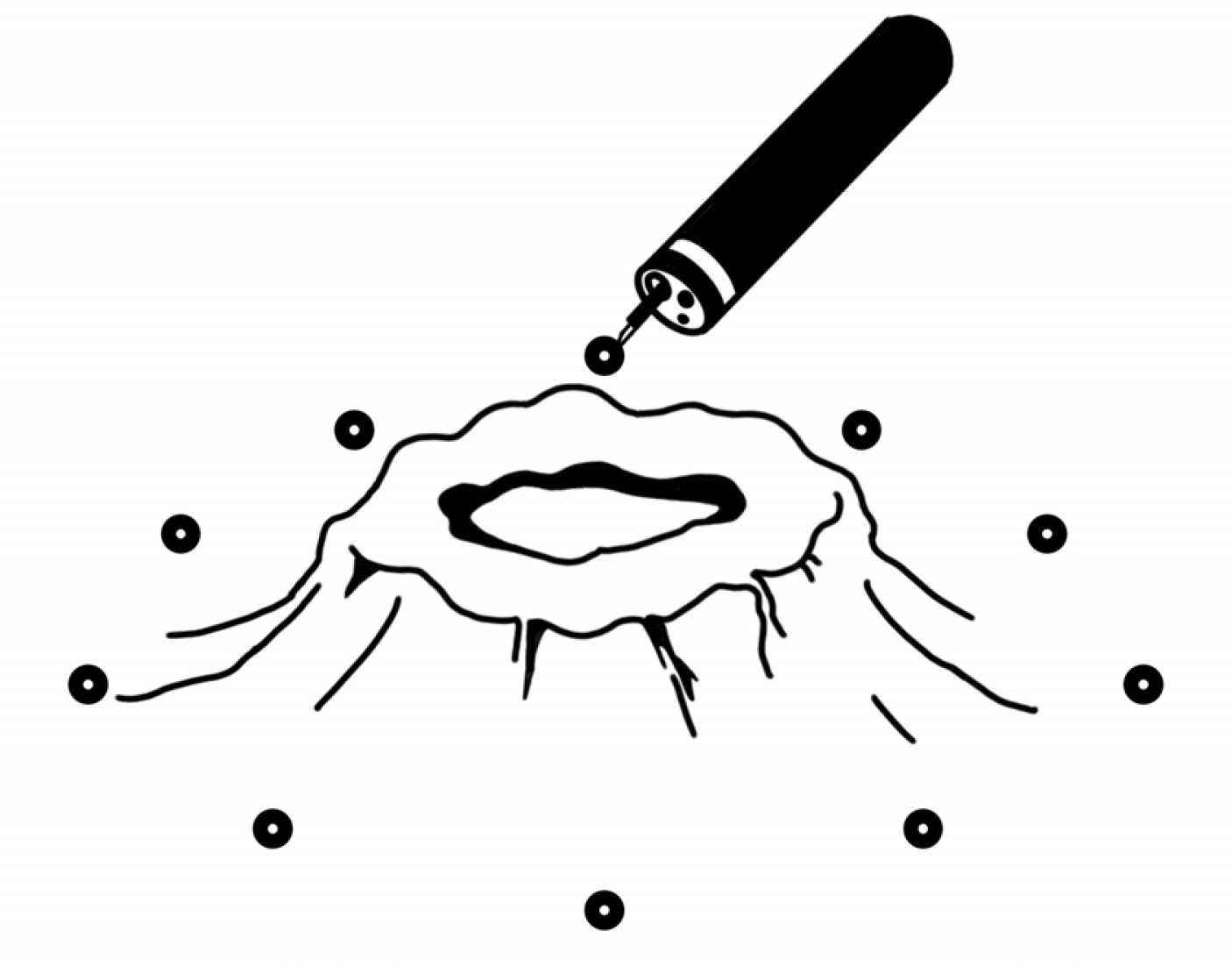
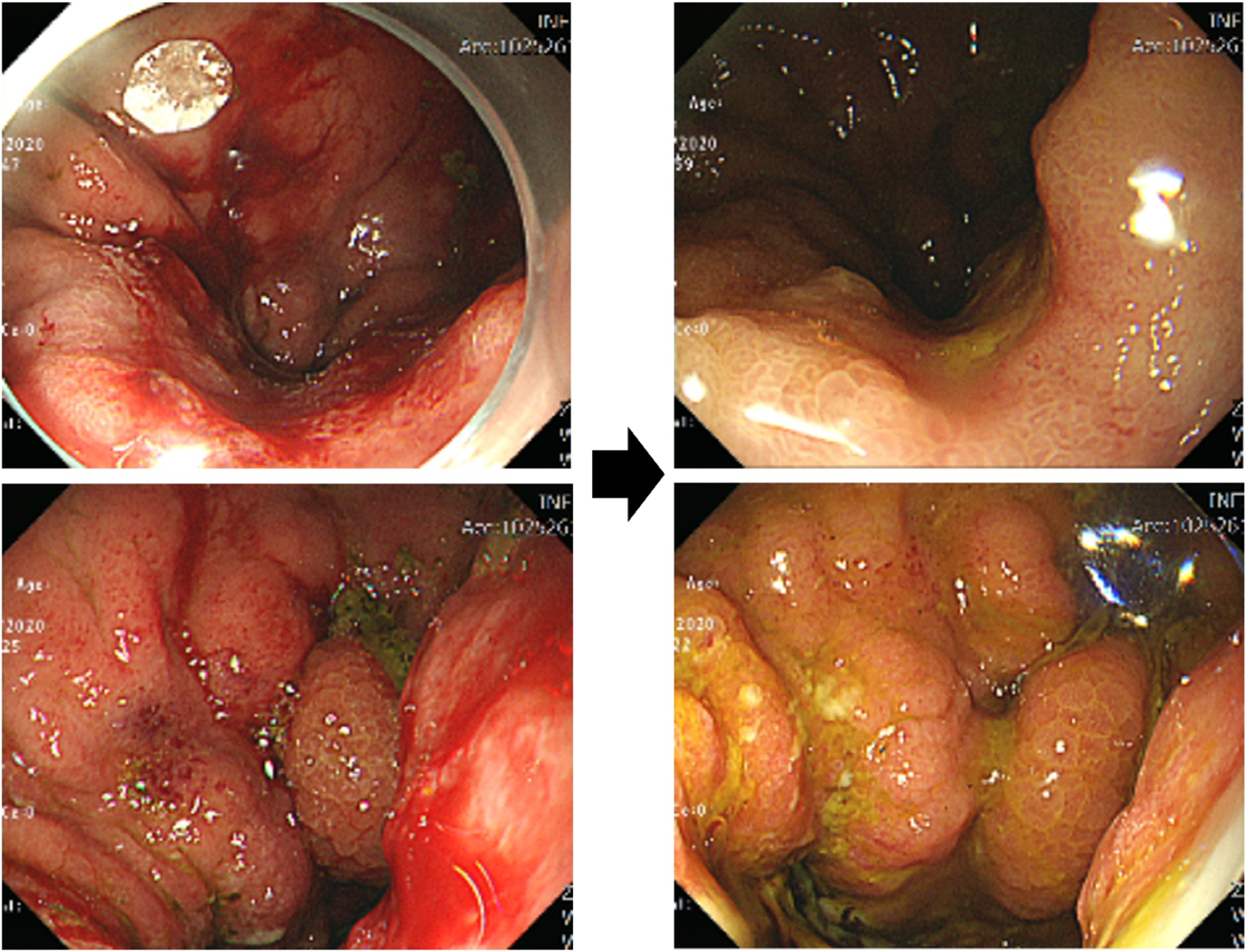
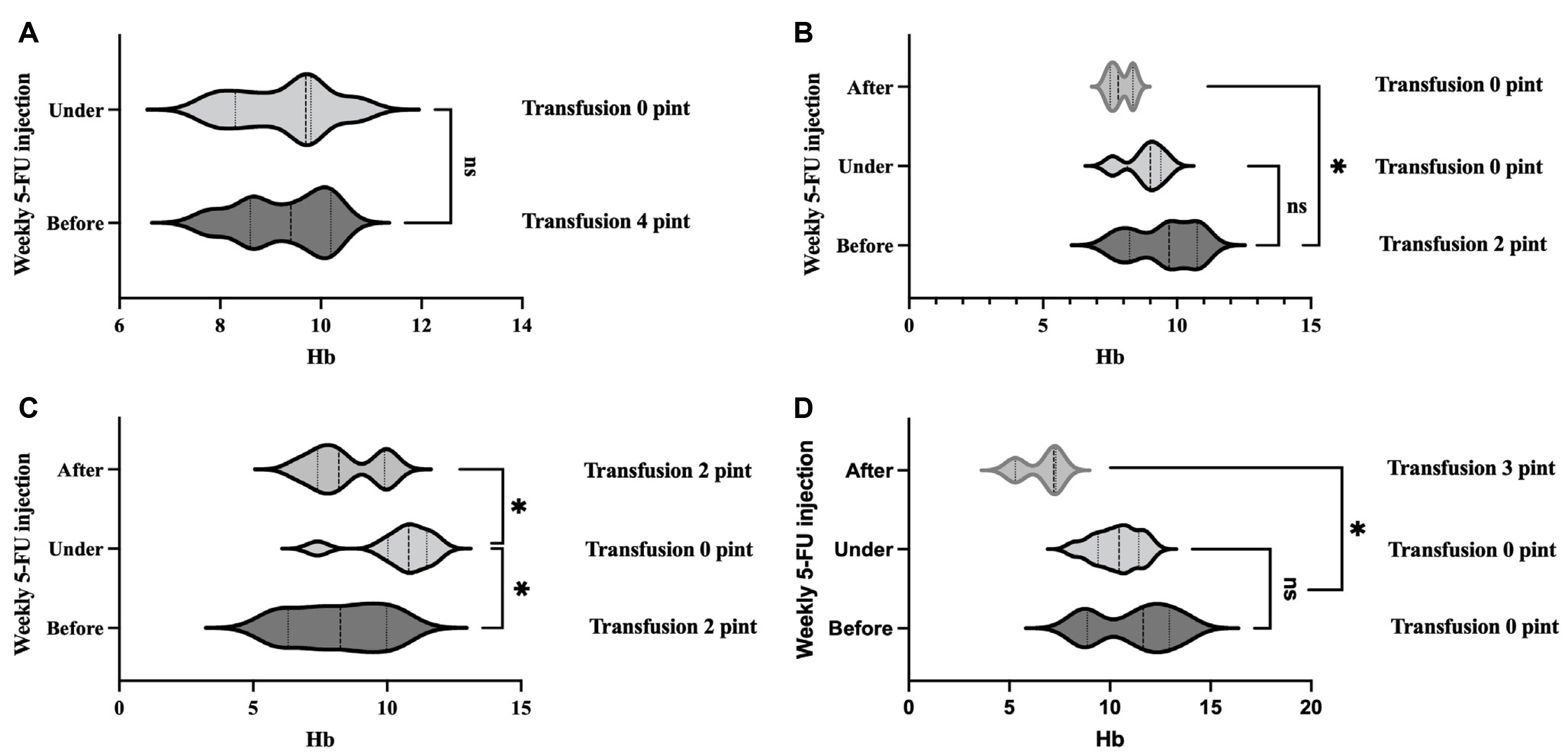
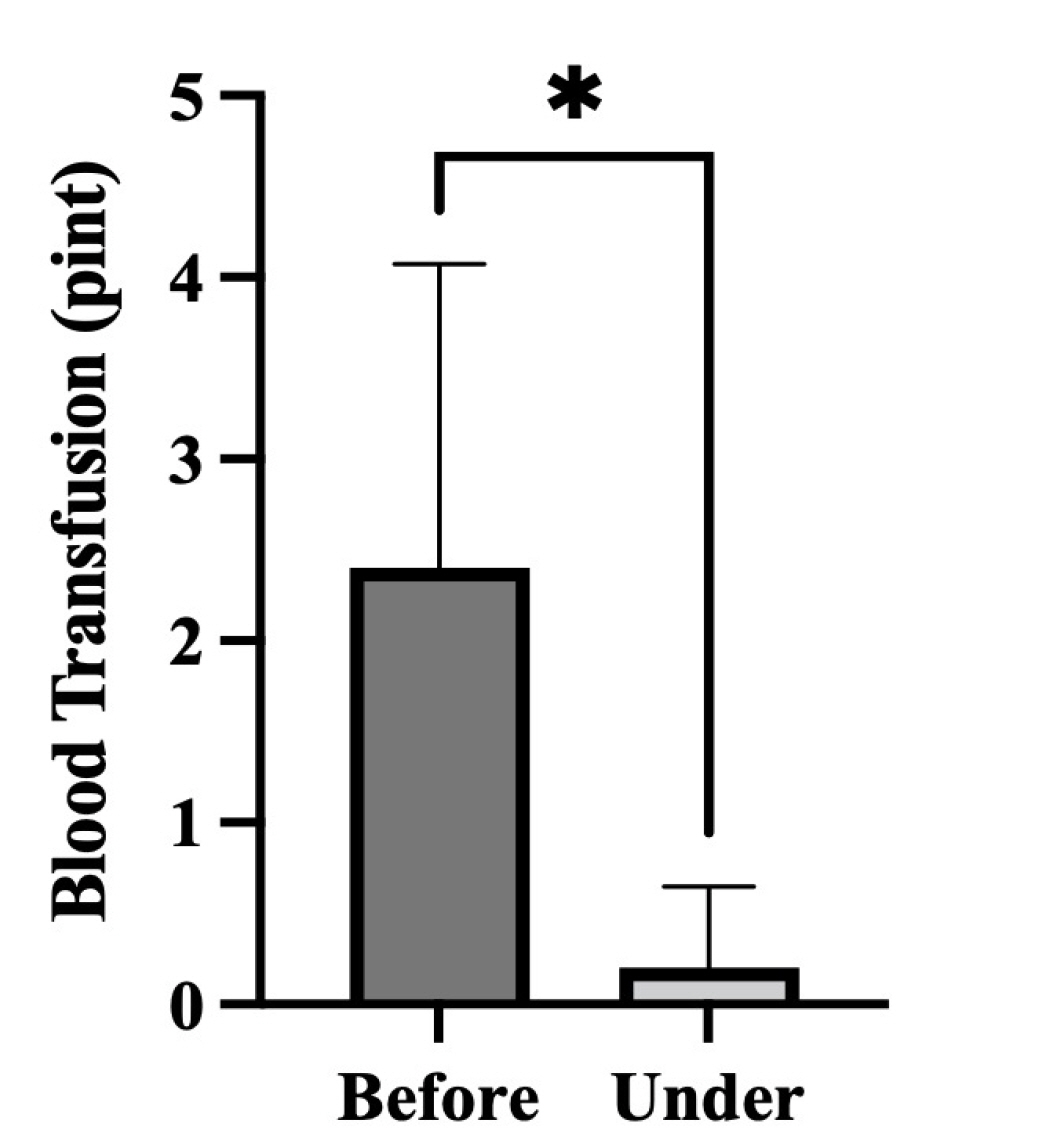
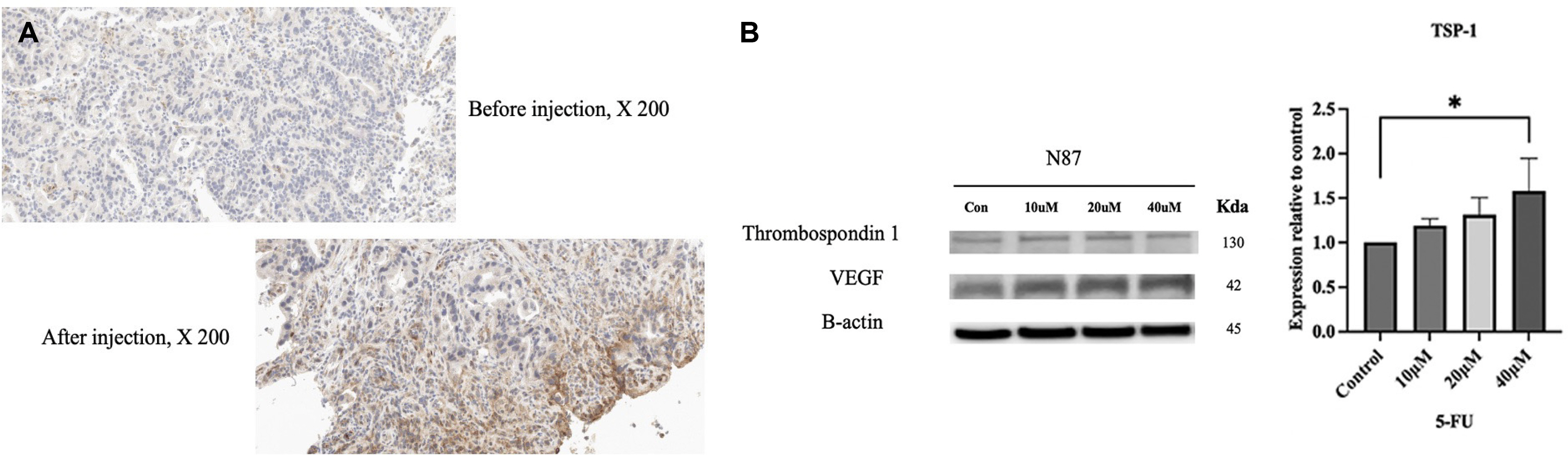
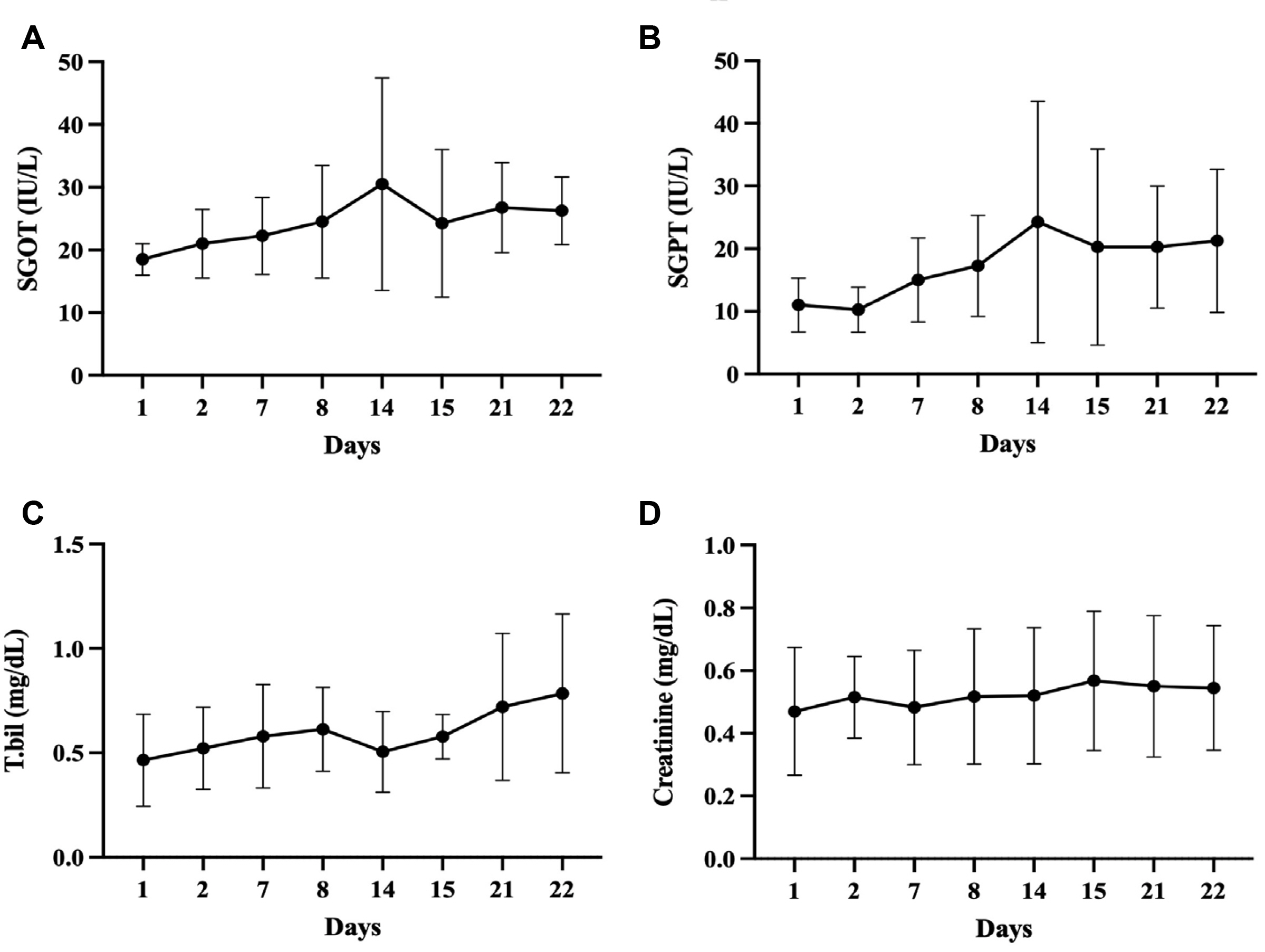
- Five-FU is a potent chemotherapeutic agent for suppressing endothelial cell growth. The purpose of this study was to investigate the usefulness of local peritumor injection of 5-FU for patients with advanced gastric cancer (AGC) for the prevention of anemia. Between January 2020 and January 2022, patients aged 18 years or older with AGC and moderate anemia were included. A total of 200 mg of 5-FU was injected per session at ten points of the lesion (20 mg at each point) every 7 days for 4 to 12 weeks. Patients received a blood test for toxicity at every cycle. From one of these patients, endoscopic biopsy specimens were taken from gastric cancer before and after injecting 5-FU for immunostaining. A total of five AGC patients participated in this study. For most patients, hemoglobin levels were maintained without transfusions during 5-FU injection, and expression levels of thrombospondin-1 was increased after injection compared to those before injection. Blood test results during 5-FU injection showed no significant change in serum glutamic oxalacetic transaminase/glutamic pyruvic transaminase, total bilirubin, or creatinine level. The results of this study showed the possibility of local peritumor 5-FU injection as a treatment for relieving anemia of patients with gastric cancer.
Keyword
Figure
Reference
-
1. Jung KW, Park S, Kong HJ, et al. 2010; Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 25:1113–1121. DOI: 10.3346/jkms.2010.25.8.1113. PMID: 20676319. PMCID: PMC2908777.
Article2. Groopman JE, Itri LM. 1999; Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 91:1616–1634. DOI: 10.1093/jnci/91.19.1616. PMID: 10511589.
Article3. Bermejo F, García-López S. 2009; A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 15:4638–4643. DOI: 10.3748/wjg.15.4638. PMID: 19787826. PMCID: PMC2754511.
Article4. Clarke H, Pallister CJ. 2005; The impact of anaemia on outcome in cancer. Clin Lab Haematol. 27:1–13. DOI: 10.1111/j.1365-2257.2004.00664.x. PMID: 15686502.
Article5. Ludwig H, Strasser K. 2001; Symptomatology of anemia. Semin Oncol. 28(2 Suppl 8):7–14. DOI: 10.1053/sonc.2001.25391. PMID: 11395846.
Article6. Ludwig H, Müldür E, Endler G, Hübl W. 2013; Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 24:1886–1892. DOI: 10.1093/annonc/mdt118. PMID: 23567147. PMCID: PMC3690908.
Article7. Holzner B, Kemmler G, Greil R, et al. 2002; The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 13:965–973. DOI: 10.1093/annonc/mdf122. PMID: 12123343.
Article8. Kondoh C, Shitara K, Nomura M, et al. 2015; Efficacy of palliative radiotherapy for gastric bleeding in patients with unresectable advanced gastric cancer: a retrospective cohort study. BMC Palliat Care. 14:37. DOI: 10.1186/s12904-015-0034-y. PMID: 26238344. PMCID: PMC4524128.
Article9. Asakura H, Hashimoto T, Harada H, et al. 2011; Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 137:125–130. DOI: 10.1007/s00432-010-0866-z. PMID: 20336314.
Article10. Pereira J, Phan T. 2004; Management of bleeding in patients with advanced cancer. Oncologist. 9:561–570. DOI: 10.1634/theoncologist.9-5-561. PMID: 15477642.
Article11. Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. 2012; Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 23:1954–1962. DOI: 10.1093/annonc/mds112. PMID: 22575608.
Article12. Ohta H, Takagi K, Noguchi Y, et al. 1984; Endoscopic intramural injection of anti-neoplastic emulsion. Gan. 75:641–649.13. Yamada K, Murphy GP, Douglass HO Jr. 1979; Comparison of intramural 5-fluorouracil and more conventional routes of drug administration on concentrations in gastric regional lymph nodes: a potential for trans-endoscopic adjuvant chemotherapy. J Surg Oncol. 11:341–349. DOI: 10.1002/jso.2930110409. PMID: 109704.
Article14. Robles-Jara C, Robles-Medranda C. 2010; Endoscopic chemotherapy with 5-fluorouracil in advanced gastric cancer. J Gastrointest Cancer. 41:75–78. DOI: 10.1007/s12029-009-9112-9. PMID: 19943195.
Article15. Zhang Y, Sun M, Huang G, et al. 2017; Maintenance of antiangiogenic and antitumor effects by orally active low-dose capecitabine for long-term cancer therapy. Proc Natl Acad Sci U S A. 114:E5226–E5235. DOI: 10.1073/pnas.1705066114. PMID: 28607065. PMCID: PMC5495268.
Article16. Ooyama A, Oka T, Zhao HY, Yamamoto M, Akiyama S, Fukushima M. 2008; Anti-angiogenic effect of 5-fluorouracil-based drugs against human colon cancer xenografts. Cancer Lett. 267:26–36. DOI: 10.1016/j.canlet.2008.03.008. PMID: 18420342.
Article17. Oken MM, Creech RH, Tormey DC, et al. 1982; Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 5:649–655. DOI: 10.1097/00000421-198212000-00014. PMID: 7165009.
Article18. Cwikiel M, Eskilsson J, Albertsson M, Stavenow L. 1996; The influence of 5-fluorouracil and methotrexate on vascular endothelium. An experimental study using endothelial cells in the culture. Ann Oncol. 7:731–737. DOI: 10.1093/oxfordjournals.annonc.a010723. PMID: 8905032.
Article19. Lawler PR, Lawler J. 2012; Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2:a006627. DOI: 10.1101/cshperspect.a006627. PMID: 22553494. PMCID: PMC3331684.
Article20. Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. 2001; Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 98:12485–12490. DOI: 10.1073/pnas.171460498. PMID: 11606713. PMCID: PMC60080.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy with Five-Day Continuous Infusion of 5-Fluorouracil (5-FU) Plus Cisplatin for Advanced Gastric Cancer; Significance of 5-FU Concentration Monitoring
- Chemotherapy for Advanced Gastric Cancer: Slow but Further Progress
- Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer
- Distribution of 5-Fluorouracil-14C in Body Tissues after Systemic and Regional Administration in Gastric Cancer
- Combination Chemotherapy with 5-Fluorouracil and Heptaplatin as First-line Treatment in Patients with Advanced Gastric Cancer