J Korean Med Sci.
2022 Dec;37(49):e353. 10.3346/jkms.2022.37.e353.
How We Have Treated Severe to Critically Ill Patients With Coronavirus Disease 2019 in Korea
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2537047
- DOI: http://doi.org/10.3346/jkms.2022.37.e353
Abstract
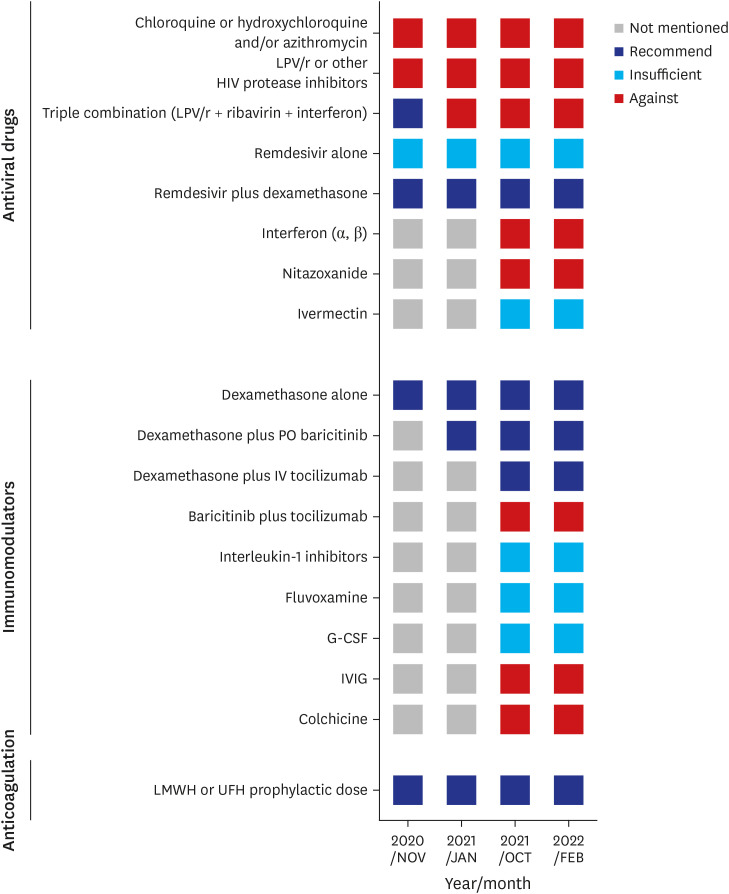
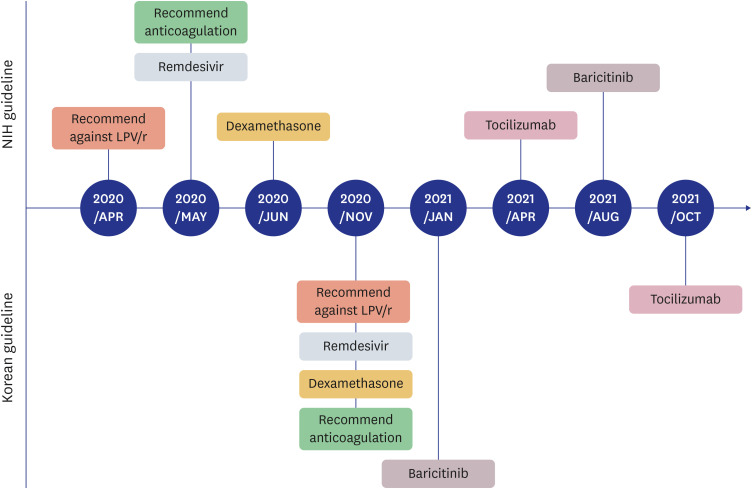
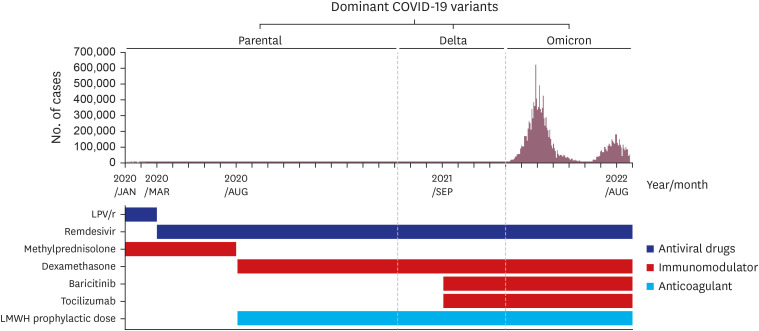
- Since 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide, and the coronavirus disease 2019 (COVID-19) pandemic currently continues. In response to this unprecedented pandemic, several researchers and medical staff have struggled to find appropriate treatments for COVID-19. Patients with mild symptoms can recuperate with symptomatic care, however establishing treatment for severe to critically ill patients who can have a high mortality has been essential. Accordingly, the guidelines for COVID-19 treatment have evolved through numerous trials and errors and have been relatively well established to date. In the Republic of Korea, several evidence-based guidelines for COVID-19 treatment were released and revised, reflecting various research and regional medical conditions. To date, approximately 3 years after the beginning of the COVID-19 pandemic, we are reflecting on the changes in the guidelines thus far and have summarized the treatment experience of severe to critically ill patients with COVID-19. The Korean guidelines for COVID-19 treatment have been updated continuously as the National Institutes of Health (NIH) guidelines have changed. Dexamethasone is currently used as the backbone for the treatment of severe to critically ill patients with COVID-19, and remdesivir, baricitinib, and tocilizumab can be added depending on a patient’s situation. In addition, venous thromboembolism prophylaxis is one of the important adjunctive therapies for patients with severe COVID-19. In the clinical field, treatment of severely ill patients with COVID-19 based on guidelines is widely practiced by medical staff and established currently.
Figure
Cited by 2 articles
-
Risk Factors for the Prescription of Ineffective Antiviral Candidates for COVID-19 During the Early Pandemic Period in Korea
Eunyoung Lee, Seungyeon Kim, Sun Young Lee, Joo Jeong, Jihwan Bang, Juhwan Oh, Sang Do Shin, Nam Joong Kim, Pyoeng Gyun Choe, Myoung-don Oh
J Korean Med Sci. 2023;38(36):e280. doi: 10.3346/jkms.2023.38.e280.Adverse Drug Events Associated With Remdesivir in Real-World Hospitalized Patients With COVID-19, Including Vulnerable Populations: A Retrospective Multicenter Study
Hyein Kang, Chang Kyung Kang, Jae Hyoung Im, Yoonsook Cho, Dong Yoon Kang, Ju-Yeun Lee
J Korean Med Sci. 2023;38(44):e346. doi: 10.3346/jkms.2023.38.e346.
Reference
-
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization website. Updated 2022. Accessed October 23, 2022. https://covid19.who.int/info .2. Adjei P, Afriyie-Mensah J, Ganu VJ, Puplampu P, Opoku-Asare B, Dzefi-Tettey K, et al. Clinical characteristics of COVID-19 patients admitted at the Korle-Bu Teaching Hospital, Accra, Ghana. Ghana Med J. 2020; 54(4):Suppl. 33–38. PMID: 33976439.3. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID: 32242348.4. Kim M, Yoo JR, Heo ST, Lee HR, Oh H. Clinical characteristics and risk factors for severe disease of coronavirus disease 2019 in a low case fatality rate region in Korea. Infect Chemother. 2021; 53(4):718–729. PMID: 34951535.5. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated 2022. Accessed October 23, 2022. https://www.covid19treatmentguidelines.nih.gov/ .6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323(13):1239–1242. PMID: 32091533.7. Shin HS. Empirical treatment and prevention of COVID-19. Infect Chemother. 2020; 52(2):142–153. PMID: 32476308.8. National Medical Center – Office for the Central Infectious Disease Hospital – National Institute of Infectious Diseases. Clinical Practice Guideline for Coronavirus disease 2019 (COVID-19). Updated 2022. Accessed October 23, 2022. https://www.nmc.or.kr/icd/bbs/B0000070/list.do?menuNo=1300032 .9. Kim SB, Kim J, Huh K, Choi WS, Kim YJ, Joo EJ, et al. Korean Society of Infectious Diseases/national evidence-based healthcare collaborating agency recommendations for anti-SARS-CoV-2 monoclonal antibody treatment of patients with COVID-19. Infect Chemother. 2021; 53(2):395–403. PMID: 34216134.10. Kim SB, Ryoo S, Huh K, Joo EJ, Kim YJ, Choi WS, et al. Revised Korean Society of Infectious Diseases/national evidence-based healthcare collaborating agency guidelines on the treatment of patients with COVID-19. Infect Chemother. 2021; 53(1):166–219. PMID: 34409790.11. National Evidence based Healthcare Collaborating Agency – Korean Academy of Medical Sciences (NECA-KAMS). Coronavirus disease 2019 (COVID-19). Living Guideline. Updated 2022. Accessed October 23, 2022. https://www.neca.re.kr/lay1/bbs/S1T11C174/F/58/list.do .12. Kim SB, Huh K, Heo JY, Joo EJ, Kim YJ, Choi WS, et al. Interim guidelines on antiviral therapy for COVID-19. Infect Chemother. 2020; 52(2):281–304. PMID: 32342676.13. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004; 323(1):264–268. PMID: 15351731.14. Hong KS, Jang JG, Hur J, Lee JH, Kim HN, Lee W, et al. Early hydroxychloroquine administration for rapid severe acute respiratory syndrome coronavirus 2 eradication. Infect Chemother. 2020; 52(3):396–402. PMID: 32757497.15. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020; 383(21):2030–2040. PMID: 33031652.16. Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild COVID-19: a randomized-controlled trial. Clin Infect Dis. 2021; 73(11):e4073–e4081. PMID: 32674126.17. Horby PW, Mafham M, Bell JL, Linsell L, Staplin N, Emberson J, et al. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020; 396(10259):1345–1352. PMID: 33031764.18. Hoang T, Anh TT. Treatment options for severe acute respiratory syndrome, middle east respiratory syndrome, and coronavirus disease 2019: a review of clinical evidence. Infect Chemother. 2020; 52(3):317–334. PMID: 32869558.19. Lee C, Ahn MY, Byeon K, Choi JP, Hahm C, Kim H, et al. Clinical experience with use of remdesivir in the treatment of severe acute respiratory syndrome coronavirus 2: a case series. Infect Chemother. 2020; 52(3):369–380. PMID: 32757500.20. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19-final report. N Engl J Med. 2020; 383(19):1813–1826. PMID: 32445440.21. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395(10236):1569–1578. PMID: 32423584.22. Joo EJ, Ko JH, Kim SE, Kang SJ, Baek JH, Heo EY, et al. Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study. J Korean Med Sci. 2021; 36(11):e83. PMID: 33754512.23. Choi JY. Convalescent plasma therapy for coronavirus disease 2019. Infect Chemother. 2020; 52(3):307–316. PMID: 32989938.24. Baek AR, Choo EJ, Kim JY, Ha TS, Park SW, Shin HB, et al. A transient effect of convalescent plasma therapy in a patient with severe covonavirus disease 2019: a case report. Infect Chemother. 2022; 54(3):553–558. PMID: 35920265.25. Im JH, Nahm CH, Baek JH, Kwon HY, Lee JS. Convalescent plasma therapy in coronavirus disease 2019: a case report and suggestions to overcome obstacles. J Korean Med Sci. 2020; 35(26):e239. PMID: 32627442.26. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021; 384(8):693–704. PMID: 32678530.27. Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021; 181(1):32–40. PMID: 33080017.28. Mariette X, Hermine O, Tharaux PL, Resche-Rigon M, Steg PG, Porcher R, et al. Effectiveness of tocilizumab in patients hospitalized with COVID-19: a follow-up of the CORIMUNO-TOCI-1 randomized clinical trial. JAMA Intern Med. 2021; 181(9):1241–1243. PMID: 34028504.29. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021; 384(9):795–807. PMID: 33306283.30. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020; 18(5):1023–1026. PMID: 32338827.31. Kalil AC, Mehta AK, Patterson TF, Erdmann N, Gomez CA, Jain MK, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021; 9(12):1365–1376. PMID: 34672949.32. Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006; 354(16):1671–1684. PMID: 16625008.33. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020; 8(3):267–276. PMID: 32043986.34. Hong JY, Ko JH, Yang J, Ha S, Nham E, Huh K, et al. Severity-adjusted dexamethasone dosing and tocilizumab combination dosing and tocilizumab combination for severe COVID-19. Yonsei Med J. 2022; 63(5):430–439. PMID: 35512745.35. Ejaz A, Ahmed MM, Tasleem A, Rafay Khan Niazi M, Ahsraf MF, Ahmad I, et al. Thromboprophylaxis in intensive care unit patients: a literature review. Cureus. 2018; 10(9):e3341. PMID: 30473974.36. Peck KR. Collaborative response to COVID-19 pandemic, and development of treatment guidelines. Infect Chemother. 2021; 53(1):151–154. PMID: 34409788.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experience of Treating Critically Ill COVID-19 Patients in Daegu, South Korea
- Treatment of Critically Ill Patients with Coronavirus Disease 2019
- Characteristics of Critically Ill COVID-19 Patients in Busan, Republic of Korea
- Jugular Vein Catheterization in Critically Ill Patients with Coronavirus Disease 2019 Can Increase the Surgeon’s Exposure
- Clinical and Epidemiological Characteristics of Coronavirus Disease 2019 in the Early Stage of Outbreak