Neonatal Med.
2022 Nov;29(4):141-148. 10.5385/nm.2022.29.4.141.
First Successful Application of Preimplantation Genetic Diagnosis for Lethal Neonatal Rigidity and Multifocal Seizure Syndrome in Korea: A Case Report
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, Korea
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Genomic Medicine, Seoul National University Children’s Hospital, Seoul, Korea
- 4Department of Pediatrics, Ewha Womans University Mokdong Hospital, Seoul, Korea
- KMID: 2536660
- DOI: http://doi.org/10.5385/nm.2022.29.4.141
Abstract
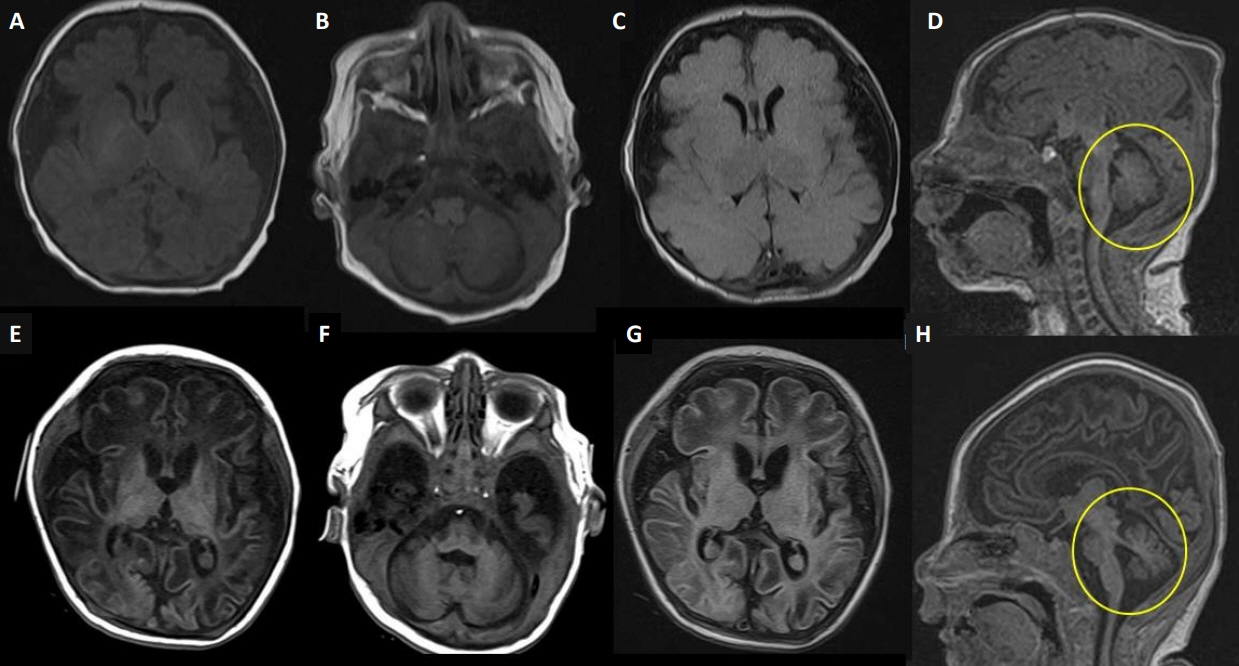
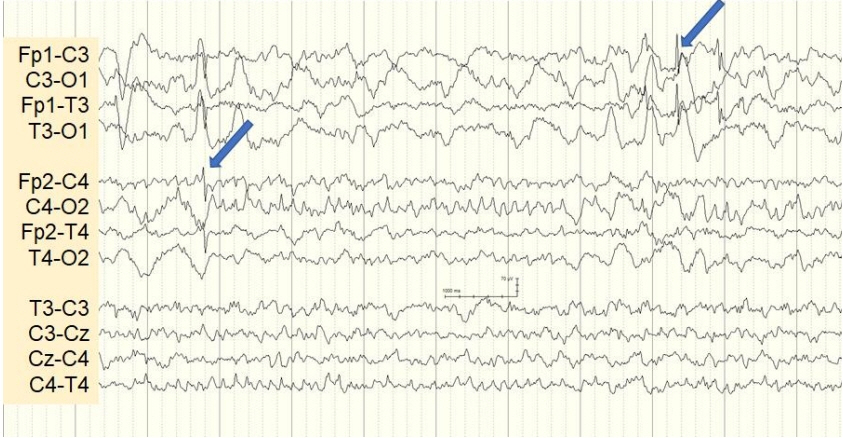
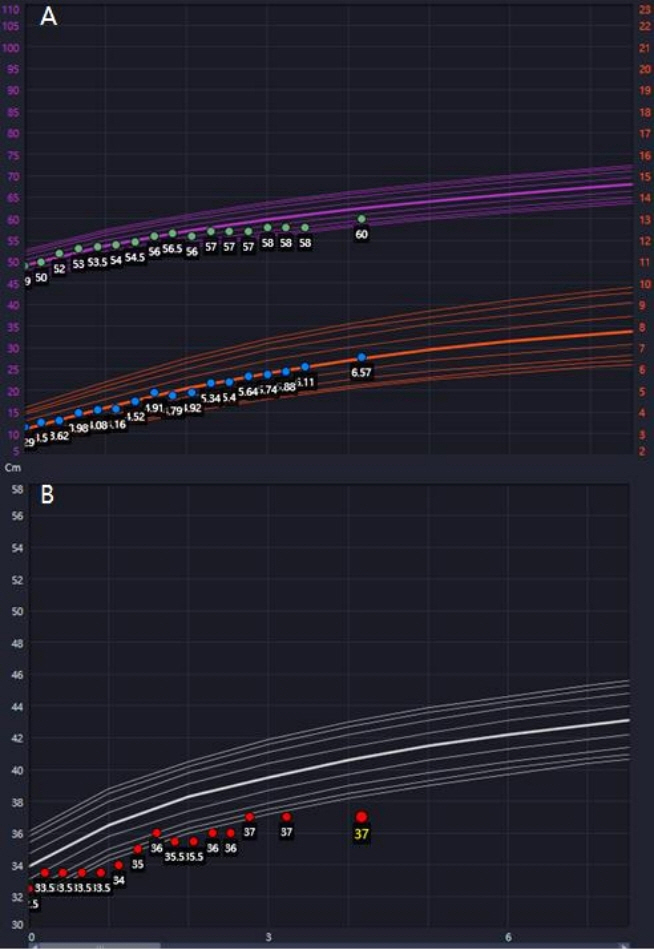
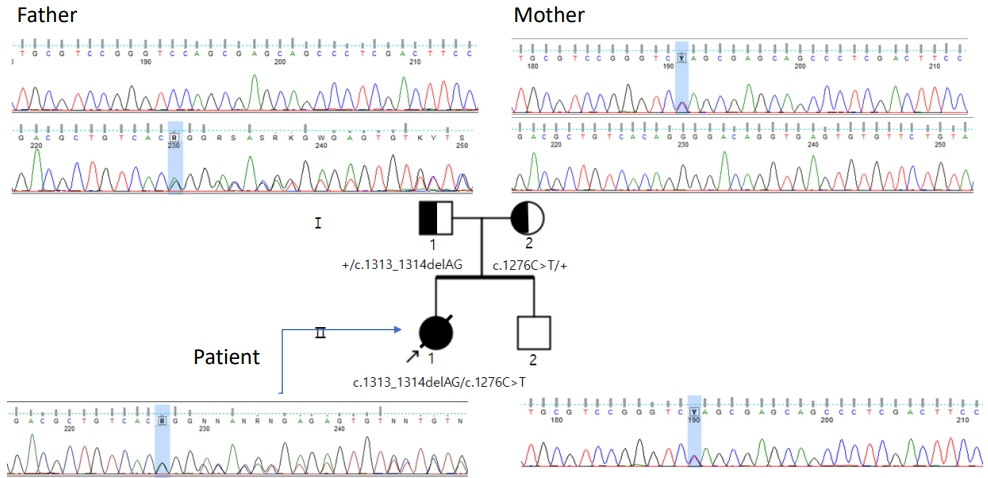
- Lethal neonatal rigidity and multifocal seizure syndrome (RMFSL) is a severe autosomal recessive epileptic encephalopathy characterized by rigidity, intractable multifocal seizures, microcephaly, apnea, and bradycardia immediately after birth. RMFSL is related to a mutation in breast cancer 1-associated ataxia telangiectasia mutated activation-1 protein (BRAT1). We report a case of a female infant born to non-consanguineous Korean parents who developed hypertonia, dysmorphic features, progressive encephalopathy with refractory seizures at birth, and worsening intermittent apnea, leading to intubation and death at 137 days of age. The initial repeated electroencephalographic findings were normal; however, a pattern of focal seizures emerged at 35 days of life. Rapid trio whole-exome sequencing revealed heterozygous mutations c.1313_1314delAG p.(Gln438Argfs*51) and c.1276C>T p. (Gln426*) in BRAT1. After genetic counseling for pregnancy planning, a preimplantation genetic diagnosis for targeted BRAT1 mutations was successfully performed, and a healthy baby was born. To our knowledge, this is the first reported case of a Korean patient with compound heterozygous mutations in BRAT1. An early and accurate genetic diagnosis can help provide timely treatment to patients and indicate the need for reproductive counseling for parents for family planning.
Figure
Reference
-
1. Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One. 2012; 7:e28936.2. Aglipay JA, Martin SA, Tawara H, Lee SW, Ouchi T. ATM activation by ionizing radiation requires BRCA1-associated BAAT1. J Biol Chem. 2006; 281:9710–8.3. Van Ommeren RH, Gao AF, Blaser SI, Chitayat DA, Hazrati LN. BRAT1 mutation: the first reported case of Chinese origin and review of the literature. J Neuropathol Exp Neurol. 2018; 77:10718.4. Saitsu H, Yamashita S, Tanaka Y, Tsurusaki Y, Nakashima M, Miyake N, et al. Compound heterozygous BRAT1 mutations cause familial Ohtahara syndrome with hypertonia and microcephaly. J Hum Genet. 2014; 59:687–90.5. So EY, Ouchi T. BRAT1 deficiency causes increased glucose metabolism and mitochondrial malfunction. BMC Cancer. 2014; 14:548.6. Pourahmadiyan A, Heidari M, Shojaaldini Ardakani H, Noorian S, Savad S. A novel pathogenic variant of BRAT1 gene causes rigidity and multifocal seizure syndrome, lethal neonatal. Int J Neurosci. 2021; 131:875–8.7. Li W, Wu S, Xu H, Zhao X, Pan Y, Huang H, et al. Novel variant in BRAT1 with the lethal neonatal rigidity and multifocal seizure syndrome. Pediatr Res. 2022; 91:565–71.8. Scheffer IE, Boysen KE, Schneider AL, Myers CT, Mehaffey MG, Rochtus AM, et al. BRAT1 encephalopathy: a recessive cause of epilepsy of infancy with migrating focal seizures. Dev Med Child Neurol. 2020; 62:1096–9.9. Colak FK, Guleray N, Azapagasi E, Yazici MU, Aksoy E, Ceylan N. An intronic variant in BRAT1 creates a cryptic splice site, causing epileptic encephalopathy without prominent rigidity. Acta Neurol Belg. 2020; 120:1425–32.10. Szymanska K, Laure-Kamionowska M, Szczaluba K, Koppolu A, Furmanek M, Kusmierska K, et al. Clinico-pathological correlation in case of BRAT1 mutation. Folia Neuropathol. 2018; 56:36271.11. Skafi O, Fawaz A, Merhi B, Jouni H, Mansour S, Harb R, et al. Rigidity with multifocal seizure syndrome, lethal neonatal in a Lebanese neonate. A rare case report. J Pediatr Disord Neonatal Care. 2018; 1:106.12. Hegde AU, Sanghvi KP, Karnavat PK, Jalan AB. BRCA1-associated ataxia telangiectasia mutated activation-1 mutation: an addition to the early infantile epileptic encephalopathy panel. J Clin Neonatol. 2017; 6:200–4.13. Celik Y, Okuyaz C, Arslankoylu AE, Ceylaner S. Lethal neonatal rigidity and multifocal seizure syndrome with a new mutation in BRAT1. Epilepsy Behav Case Rep. 2017; 8:31–2.14. Smith NJ, Lipsett J, Dibbens LM, Heron SE. BRAT1-associated neurodegeneration: intra-familial phenotypic differences in siblings. Am J Med Genet A. 2016; 170:3033–8.15. Horn D, Weschke B, Knierim E, Fischer-Zirnsak B, Stenzel W, Schuelke M, et al. BRAT1 mutations are associated with infantile epileptic encephalopathy, mitochondrial dysfunction, and survival into childhood. Am J Med Genet A. 2016; 170:2274–81.16. van de Pol LA, Wolf NI, van Weissenbruch MM, Stam CJ, Weiss JM, Waisfisz Q, et al. Early-onset severe encephalopathy with epilepsy: the BRAT1 gene should be added to the list of causes. Neuropediatrics. 2015; 46:392–400.17. Straussberg R, Ganelin-Cohen E, Goldberg-Stern H, Tzur S, Behar DM, Smirin-Yosef P, et al. Lethal neonatal rigidity and multifocal seizure syndrome: report of another family with a BRAT1 mutation. Eur J Paediatr Neurol. 2015; 19:240–2.18. Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012; 4:154ra135.19. Shellhaas RA, deVeber G, Bonkowsky JL; Child Neurology Society Research Committee. Gene-targeted therapies in pediatric neurology: challenges and opportunities in diagnosis and delivery. Pediatr Neurol. 2021; 125:53–7.20. Braude P, Pickering S, Flinter F, Ogilvie CM. Preimplantation genetic diagnosis. Nat Rev Genet. 2002; 3:941–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Application of Preimplantation Genetic Diagnosis (PGD)
- A Case of Recurrent Spontaneous Abortion Successfully Delivered by Using Preimplantation Genetic Diagnosis
- A Case of Successful Pregnancy in Patient with Recurrent Spontaneous Abortion by Preimplantation Genetic Diagnosis Following IVF-ET
- A Neuroleptic Malignant Syndrome Without Rigidity
- The first successful live birth following preimplantation genetic diagnosis using PCR for type 1 citrullinemia