Obstet Gynecol Sci.
2022 Nov;65(6):531-541. 10.5468/ogs.22108.
Triphenyl phosphate activates estrogen receptor α/NF-κB/ cyclin D1 signaling to stimulate cell cycle progression in human Ishikawa endometrial cancer cells
- Affiliations
-
- 1Roen Women’s Clinic, Busan, Korea
- 2Department of Obstetrics and Gynecology, Dong-A University Medical Center, College of Medicine, Dong-A University, Busan, Korea
- 3Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX, USA
- 4Department of Neurosurgery, College of Medicine, Dong-A University, Busan, Korea
- 5Samsung Jeil Woman's Clinic, Busan, Korea
- KMID: 2535961
- DOI: http://doi.org/10.5468/ogs.22108
Abstract
Objective
Triphenyl phosphate (TPHP) is one of the most commonly used organophosphorus flame retardants that may accumulate in the environment. However, its effects on human reproductive organs have not been well studied. We aimed to investigate the in vitro effects of TPHP in human Ishikawa endometrial cancer cells to elucidate how TPHP exposure disrupts intracellular signaling and cell proliferation in reproductive tissues.
Methods
Human Ishikawa endometrial cancer cells were exposed to TPHP.
Results
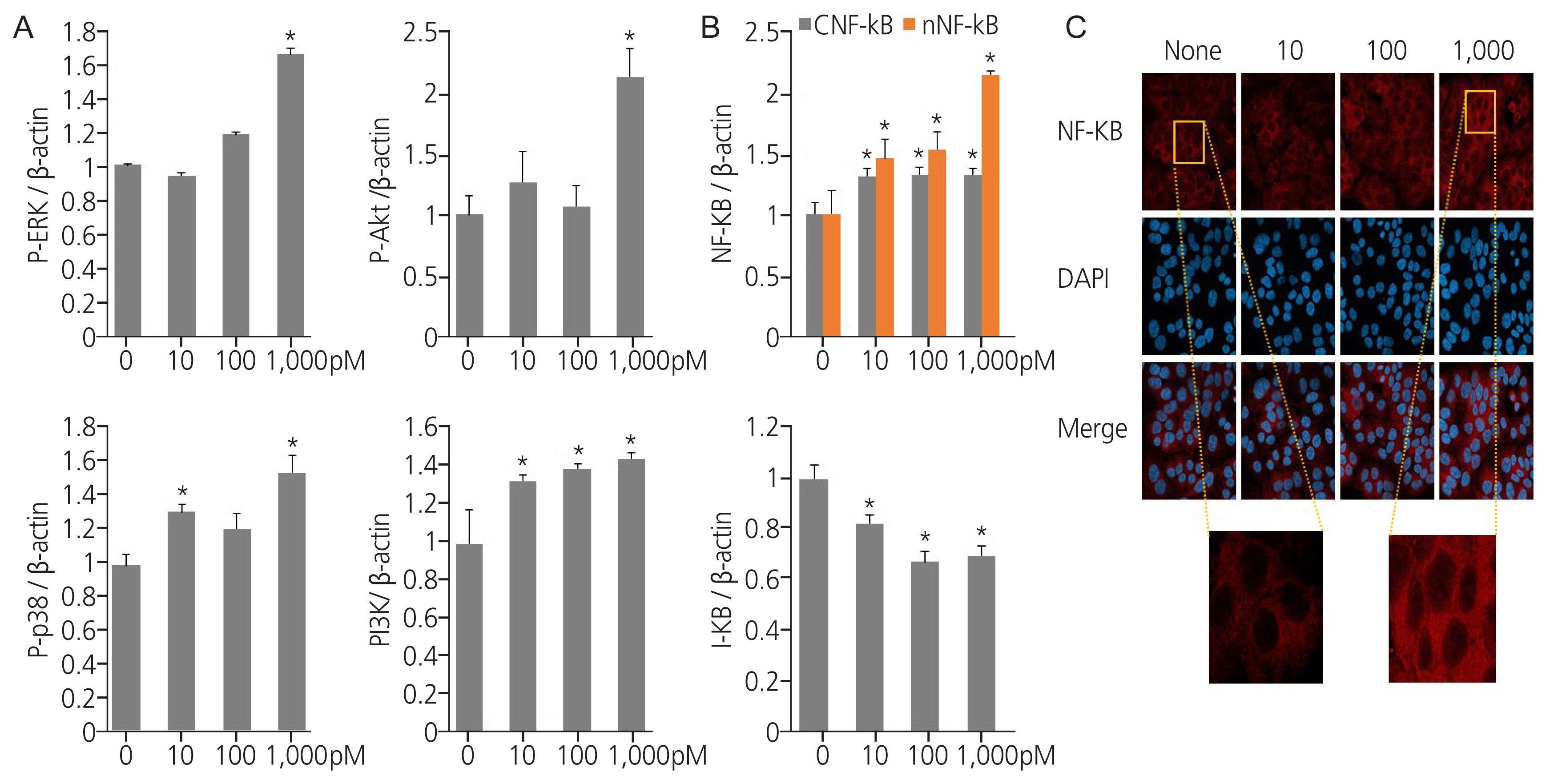
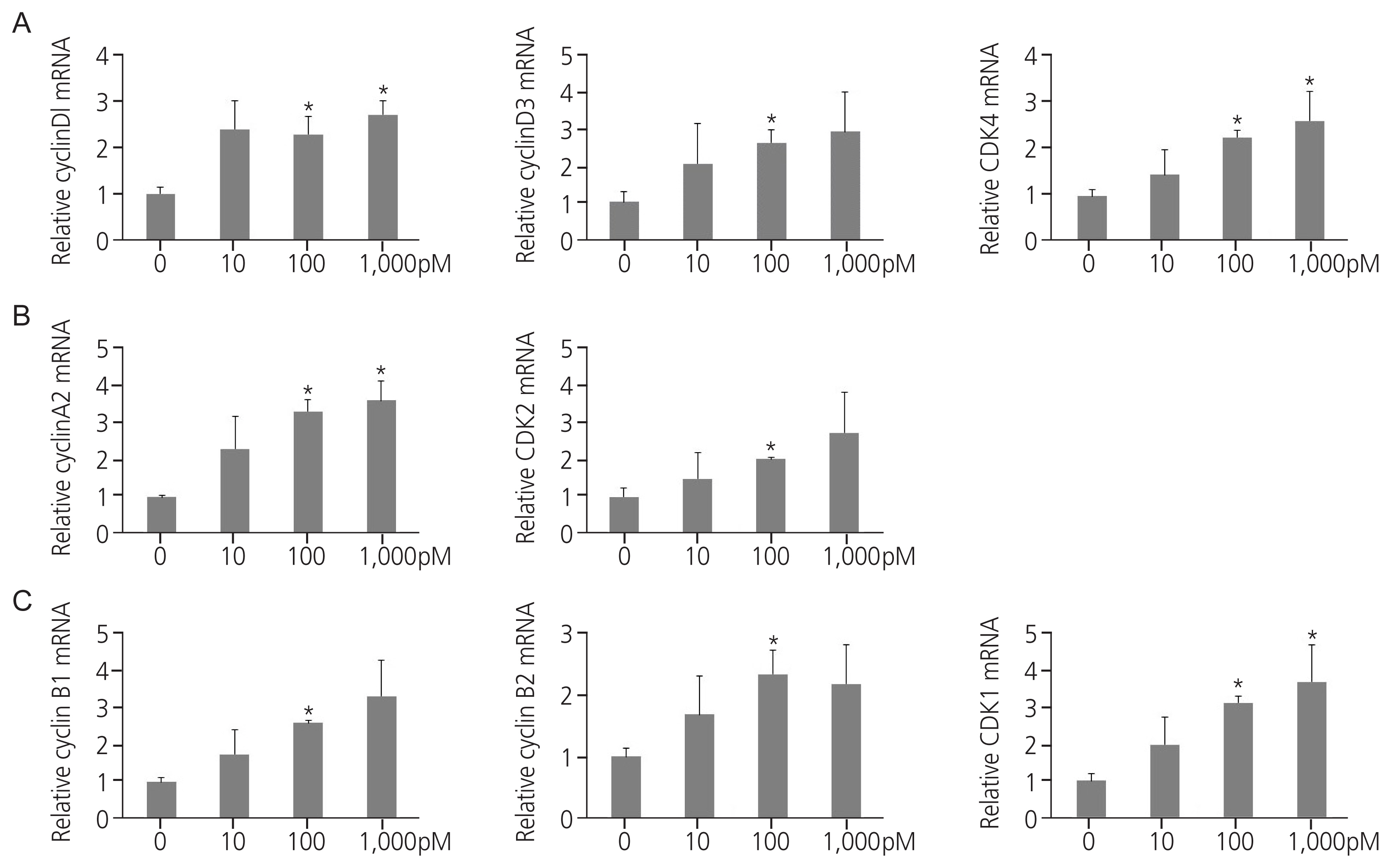
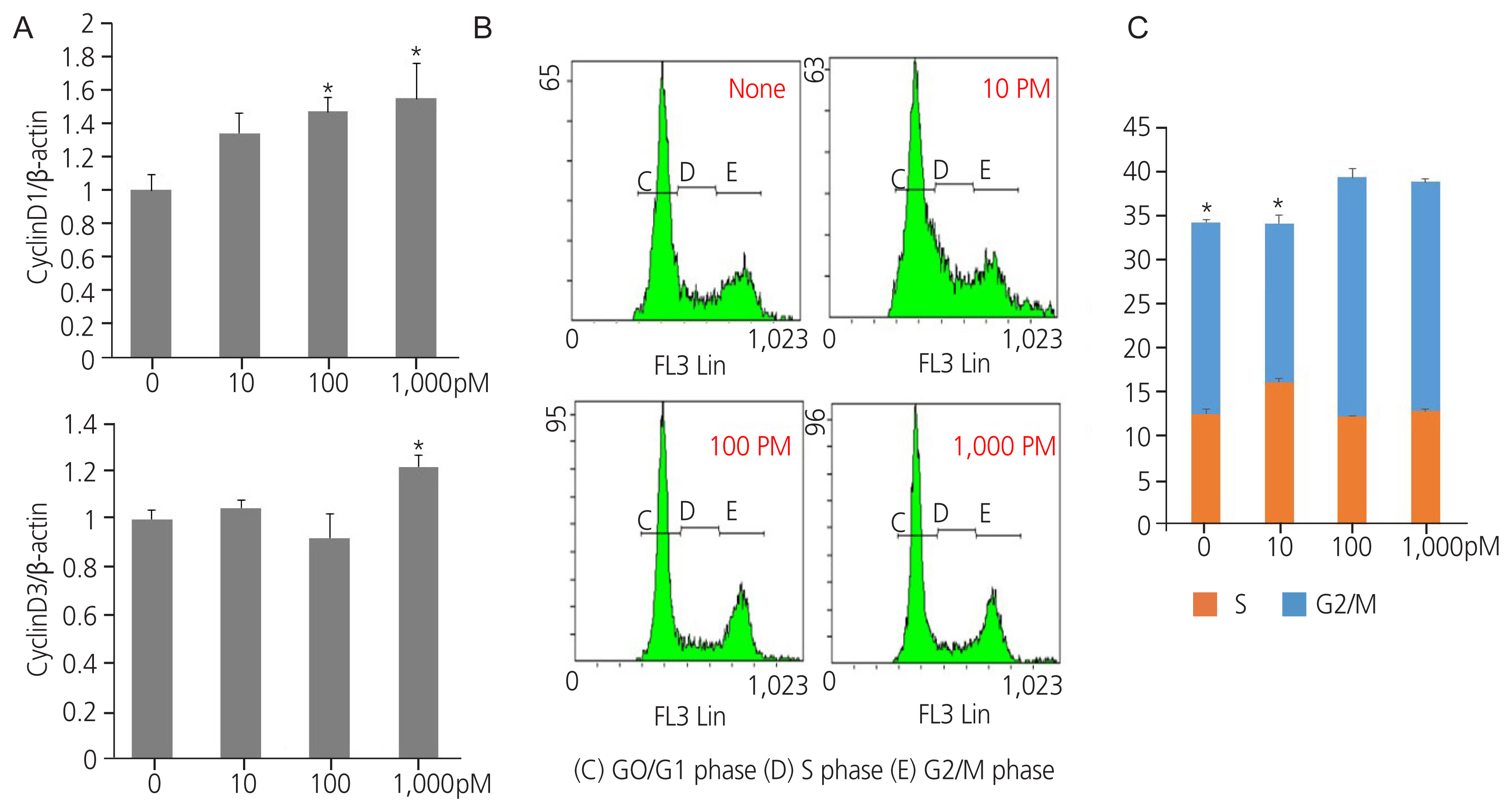
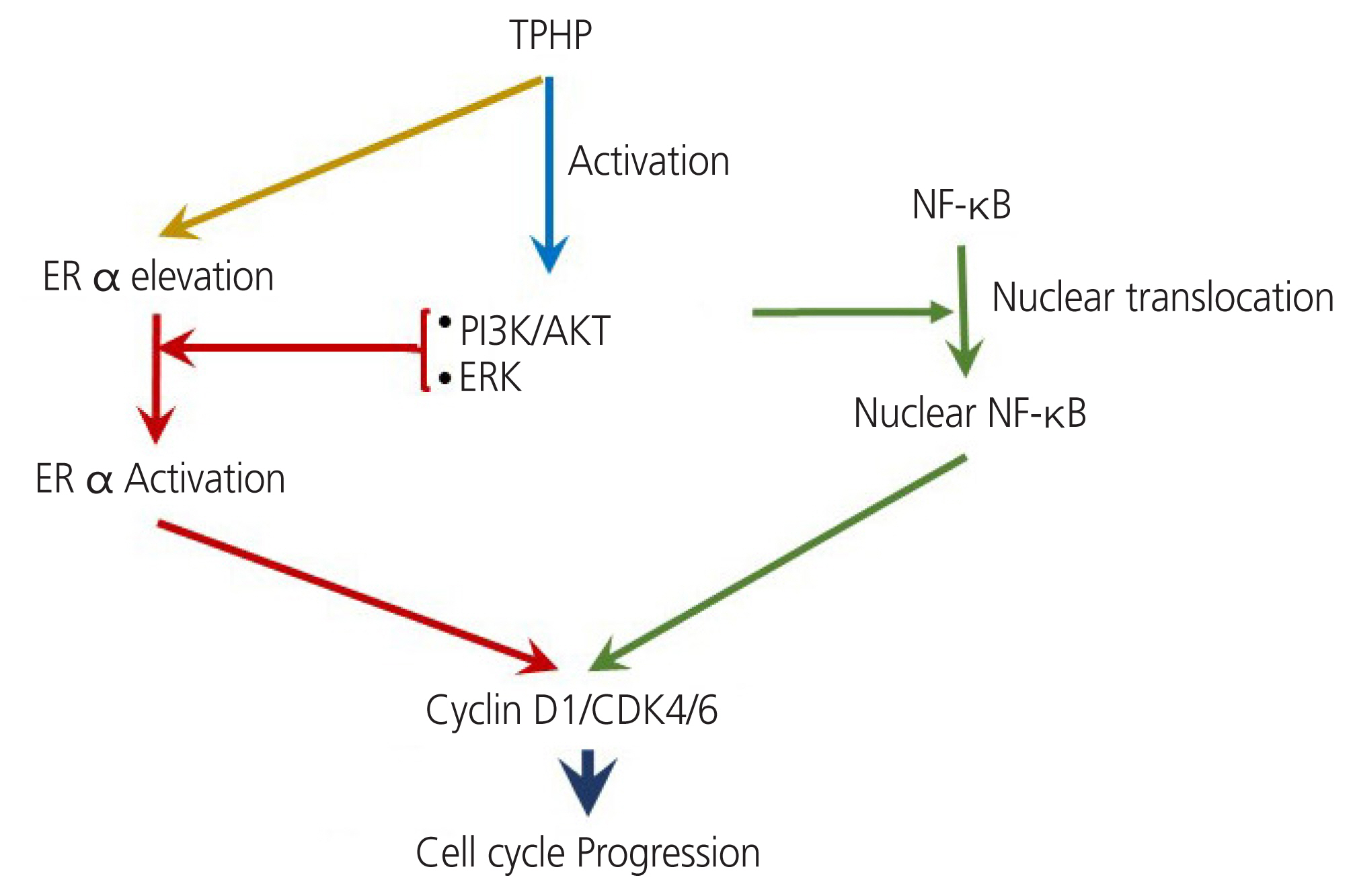
Exposure to TPHP elevated the levels of estrogen receptor (ER) α and progesterone receptor-B and reduced ER β in human Ishikawa endometrial cancer cells. TPHP stimulated phosphoinositide 3-kinase/protein kinase B and mitogenactivated protein kinase/ extracellular signal-regulated kinases 1/2 kinase signaling, which may contribute to the activation of ER function and induce nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in human Ishikawa endometrial cancer cells. Activated ER and NF-κB stimulate the expression of cyclin D1/ cyclin-dependent kinase (CDK) 4/CDK6, indicating cell cycle progression and proliferation.
Conclusion
This report may provide new information on the molecular mechanisms underlying how TPHP exposure dysregulates the cellular physiology of the human endometrium.
Keyword
Figure
Reference
-
References
1. Van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012; 88:1119–53.2. Wang W, Deng S, Li D, Ren L, Shan D, Wang B, et al. Sorption behavior and mechanism of organophosphate flame retardants on activated carbons. Chem Eng J. 2018; 332:286–92.
Article3. Zhao F, Chen M, Gao F, Shen H, Hu J. Organophosphorus flame retardants in pregnant women and their transfer to chorionic villi. Environ Sci Technol. 2017; 51:6489–97.
Article4. Wu M, Yu G, Cao Z, Wu D, Liu K, Deng S, et al. Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere. 2016; 150:465–71.
Article5. Li J, Yu N, Zhang B, Jin L, Li M, Hu M, et al. Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 2014; 54:53–61.6. Kim JW, Isobe T, Muto M, Tue NM, Katsura K, Malarvannan G, et al. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere. 2014; 116:91–7.
Article7. Hoffman K, Butt CM, Chen A, Limkakeng AT Jr, Stapleton HM. High exposure to organophosphate flame retardants in infants: associations with baby products. Environ Sci Technol. 2015; 49:14554–9.
Article8. Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, et al. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. 2016; 86:45–51.
Article9. Ospina M, Jayatilaka NK, Wong LY, Restrepo P, Calafat AM. Exposure to organophosphate flame retardant chemicals in the U.S. general population: data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int. 2018; 110:32–41.
Article10. Du Z, Zhang Y, Wang G, Peng J, Wang Z, Gao S. TPhP exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver. Sci Rep. 2016; 6:21827.11. Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (danio rerio). Aquat Toxico. 2013; 134–135:104–11.
Article12. Wang D, Zhu W, Chen L, Yan J, Teng M, Zhou Z. Neonatal triphenyl phosphate and its metabolite diphenyl phosphate exposure induce sex- and dose-dependent metabolic disruptions in adult mice. Environ Pollut. 2018; 237:10–7.
Article13. Kim S, Jung J, Lee I, Jung D, Youn H, Choi K. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat Toxicol. 2015; 160:188–96.
Article14. Li Y, Chen R, He J, Ma H, Zhao F, Tao S, et al. Triphenyl phosphate at environmental levels retarded ovary development and reduced egg production in Japanese medaka (oryzias latipes). Environ Sci Technol. 2019; 53:14709–15.15. Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol. 2012; 114–115:173–81.
Article16. Carignan CC, Mínguez-Alarcón L, Butt CM, Williams PL, Meeker JD, Stapleton HM, et al. Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environ Health Perspect. 2017; 125:087018.17. Messerlian C, Williams PL, Mínguez-Alarcón L, Carignan CC, Ford JB, Butt CM, et al. Organophosphate flame-retardant metabolite concentrations and pregnancy loss among women conceiving with assisted reproductive technology. Fertil Steril. 2018; 110:1137–44e1.
Article18. Schaefer WR, Fischer L, Deppert WR, Hanjalic-Beck A, Seebacher L, Weimer M, et al. In vitro-Ishikawa cell test for assessing tissue-specific chemical effects on human endometrium. Reprod Toxicol. 2010; 30:89–93.
Article19. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012; 33:378–455.
Article20. La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020; 16:45–57.
Article21. Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from the endocrine society. Endocrinology. 2012; 153:4097–110.
Article22. Ji X, Li N, Ma M, Rao K, Wang Z. In vitro estrogen-disrupting effects of organophosphate flame retardants. Sci Total Environ. 2020; 727:138484.
Article23. Soubry A, Hoyo C, Butt CM, Fieuws S, Price TM, Murphy SK, et al. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ Epigenet. 2017; 3:dvx003.
Article24. Kojima H, Takeuchi S, Van den Eede N, Covaci A. Effects of primary metabolites of organophosphate flame retardants on transcriptional activity via human nuclear receptors. Toxicol Lett. 2016; 245:31–9.
Article25. Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009; 94:615–22.26. Toporova L, Balaguer P. Nuclear receptors are the major targets of endocrine disrupting chemicals. Mol Cell Endocrinol. 2020; 502:110665.
Article27. Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014; 40:862–71.
Article28. Kim SC, Boese AC, Moore MH, Cleland RM, Chang L, Delafontaine P, et al. Rapid estrogen receptor-α signaling mediated by ERK activation regulates vascular tone in male and ovary-intact female mice. Am J Physiol Heart Circ Physiol. 2018; 314:H330–42.
Article29. Pierozan P, Jerneren F, Karlsson O. Perfluorooctanoic acid (PFOA) exposure promotes proliferation, migration and invasion potential in human breast epithelial cells. Arch Toxicol. 2018; 92:1729–39.
Article30. Lee HR, Hwang KA, Nam KH, Kim HC, Choi KC. Progression of breast cancer cells was enhanced by endocrine-disrupting chemicals, triclosan and octylphenol, via an estrogen receptor-dependent signaling pathway in cellular and mouse xenograft models. Chem Res Toxicol. 2014; 27:834–42.
Article31. Yu Q, Sicinska E, Geng Y, Ahnström M, Zagozdzon A, Kong Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006; 9:23–32.
Article32. Lamb J, Ladha MH, McMahon C, Sutherland RL, Ewen ME. Regulation of the functional interaction between cyclin D1 and the estrogen receptor. Mol Cell Biol. 2000; 20:8667–75.
Article33. Lamb R, Lehn S, Rogerson L, Clarke RB, Landberg G. Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle. 2013; 12:2384–94.
Article34. Mahmoodzadeh S, Fritschka S, Dworatzek E, Pham TH, Becher E, Kuehne A, et al. Nuclear factor-kappaB regulates estrogen receptor-alpha transcription in the human heart. J Biol Chem. 2009; 284:24705–14.35. Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009; 125:2863–70.36. Kasai F, Hirayama N, Ozawa M, Iemura M, Kohara A. Changes of heterogeneous cell populations in the Ishikawa cell line during long-term culture: proposal for an in vitro clonal evolution model of tumor cells. Genomics. 2016; 107:259–66.
Article37. Zhao F, Wan Y, Zhao H, Hu W, Mu D, Webster TF, et al. Levels of blood organophosphorus flame retardants and association with changes in human sphingolipid homeostasis. Environ Sci Technol. 2016; 50:8896–903.
Article38. Ding J, Xu Z, Huang W, Feng L, Yang F. Organophosphate ester flame retardants and plasticizers in human placenta in eastern China. Sci Total Environ. 2016; 554:211–7.
Article39. Canbaz D, Logiantara A, van Ree R, van Rijt LS. Immunotoxicity of organophosphate flame retardants TPHP and TDCIPP on murine dendritic cells in vitro. Chemosphere. 2017; 177:56–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between G1 Cyclins Expression and Clinical Prognostic Parameters in Endometrial Carcinoma
- TRIM29 Overexpression Promotes Proliferation and Survival of Bladder Cancer Cells through NF-κB Signaling
- Cyclin D1 Expression in Primary Breast Carcinoma: Correlation with Estrogen Receptor Status and Other Clinicopathologic Parameters
- Study of the Relationships between Cyclin D1 and Known Prognostic Factors in Breast Cancer
- Cyclin D1 Expression in Primary Breast Carcinoma: Correlation with Estrogen Receptor Status and Other Clinicopathologic Parameters