Korean J Gastroenterol.
2022 Oct;80(4):177-185. 10.4166/kjg.2022.077.
Efficacy and Safety of Endoscopic Stenting for Crohn's Disease Related Strictures: A Systematic Review and Meta-analysis
- Affiliations
-
- 1Department of Gastroenterology, Nizam's Institute of Medical Sciences, Hyderabad, India
- 2Department of Gastroenterology, Seth GS Medical College and KEM Hospital, Mumbai, India
- 3Department of Digestive Diseases and Clinical Nutrition, Tata Memorial Hospital, Mumbai, India
- KMID: 2534466
- DOI: http://doi.org/10.4166/kjg.2022.077
Abstract
- Background/Aims
Endoscopic stenting is an evolving treatment for symptomatic Crohn's strictures. Several case series and small studies have reported its efficacy. Future studies can be designed based on a systematic review of the evaluation of efficacy. Hence, this meta-analysis was conducted to assess the critical role of stents in the management of intestinal strictures associated with Crohn's disease (CD).
Methods
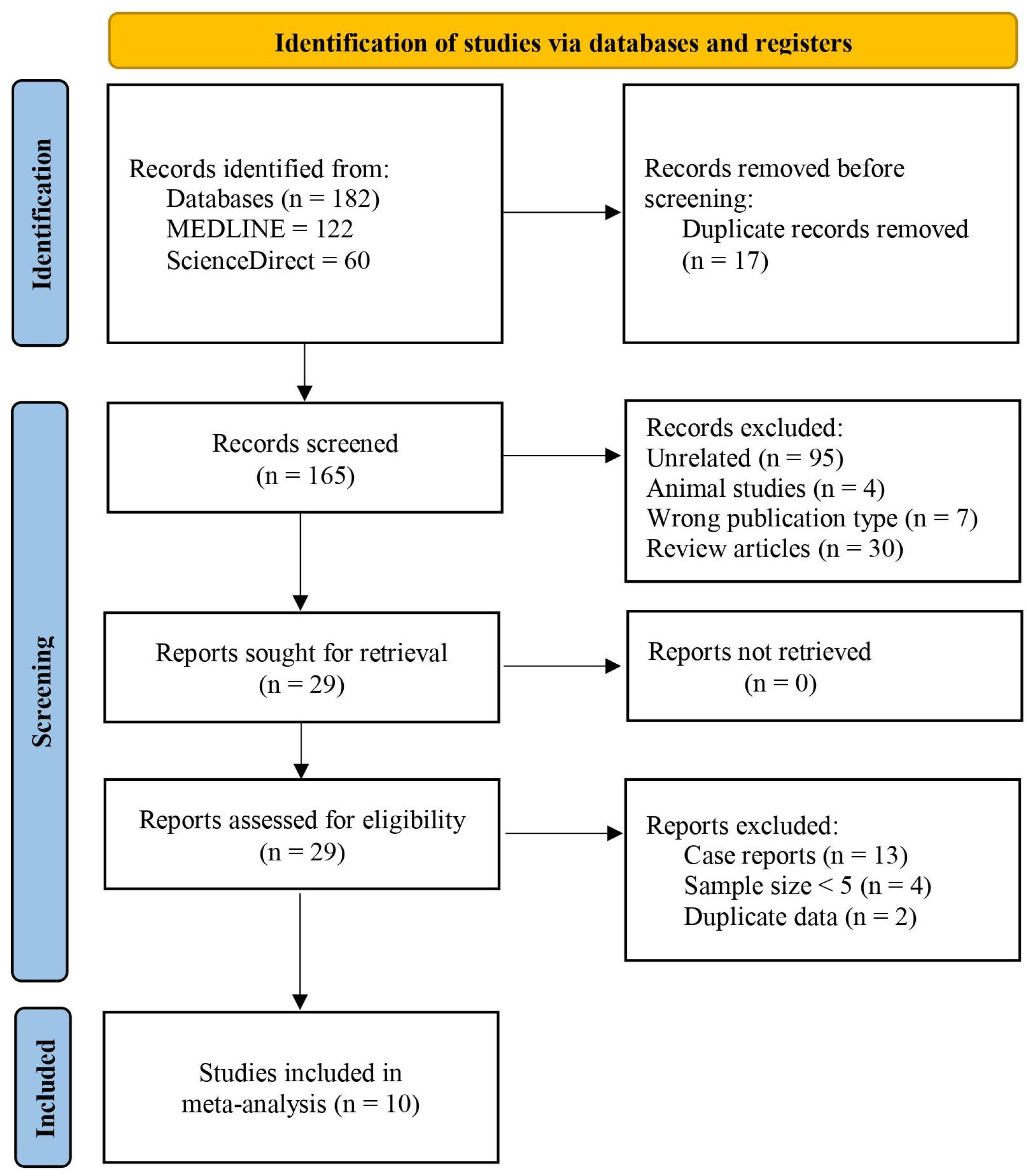
A literature search of various databases from 2000 to February 2022 was conducted for studies evaluating the outcome of stents in patients with CD-related stricture. The outcomes assessed included technical and clinical success, adverse events, symptom recurrence, and the need for a surgical resection. Pooled event rates across studies were expressed with summative statistics.
Results
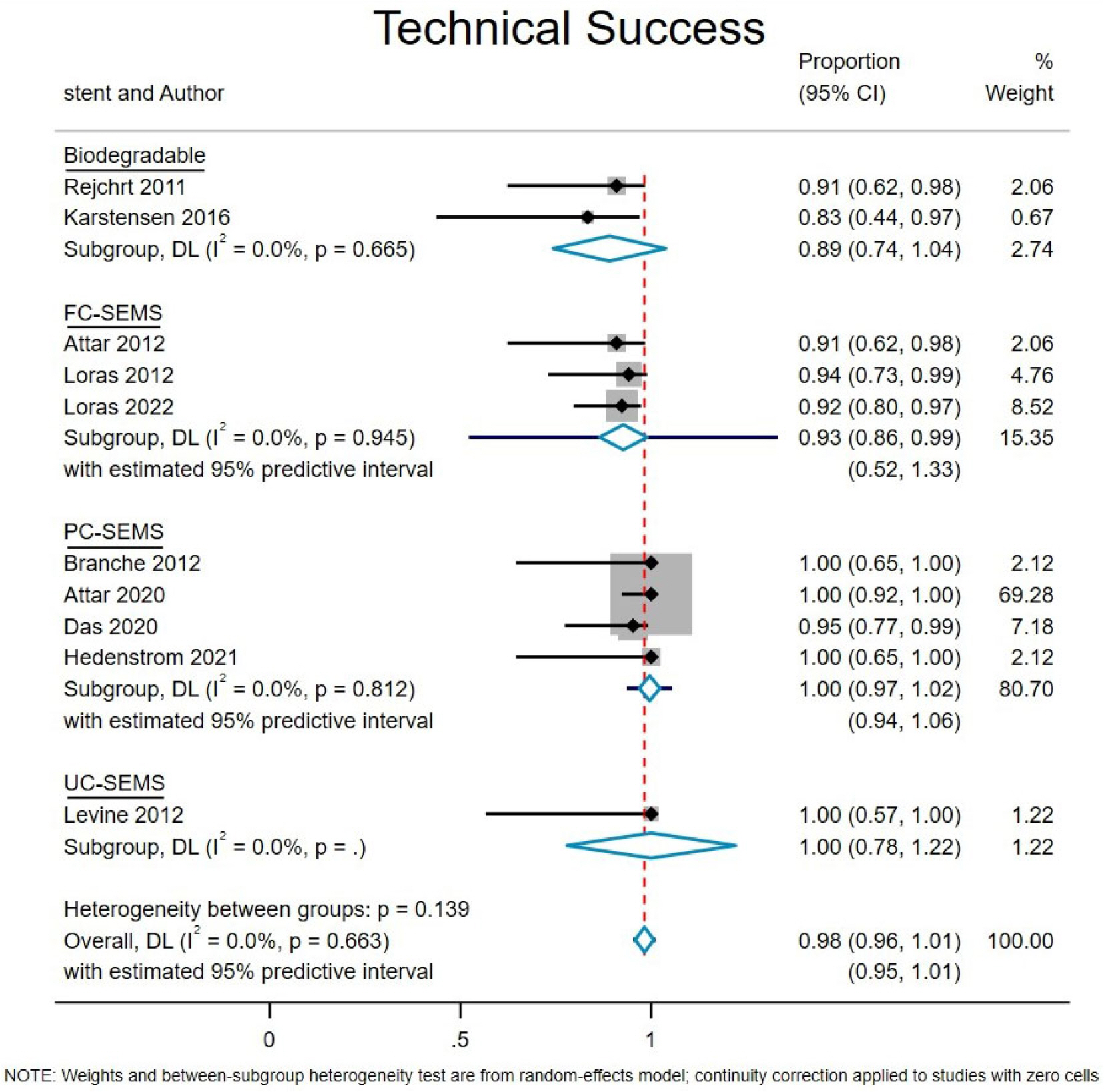
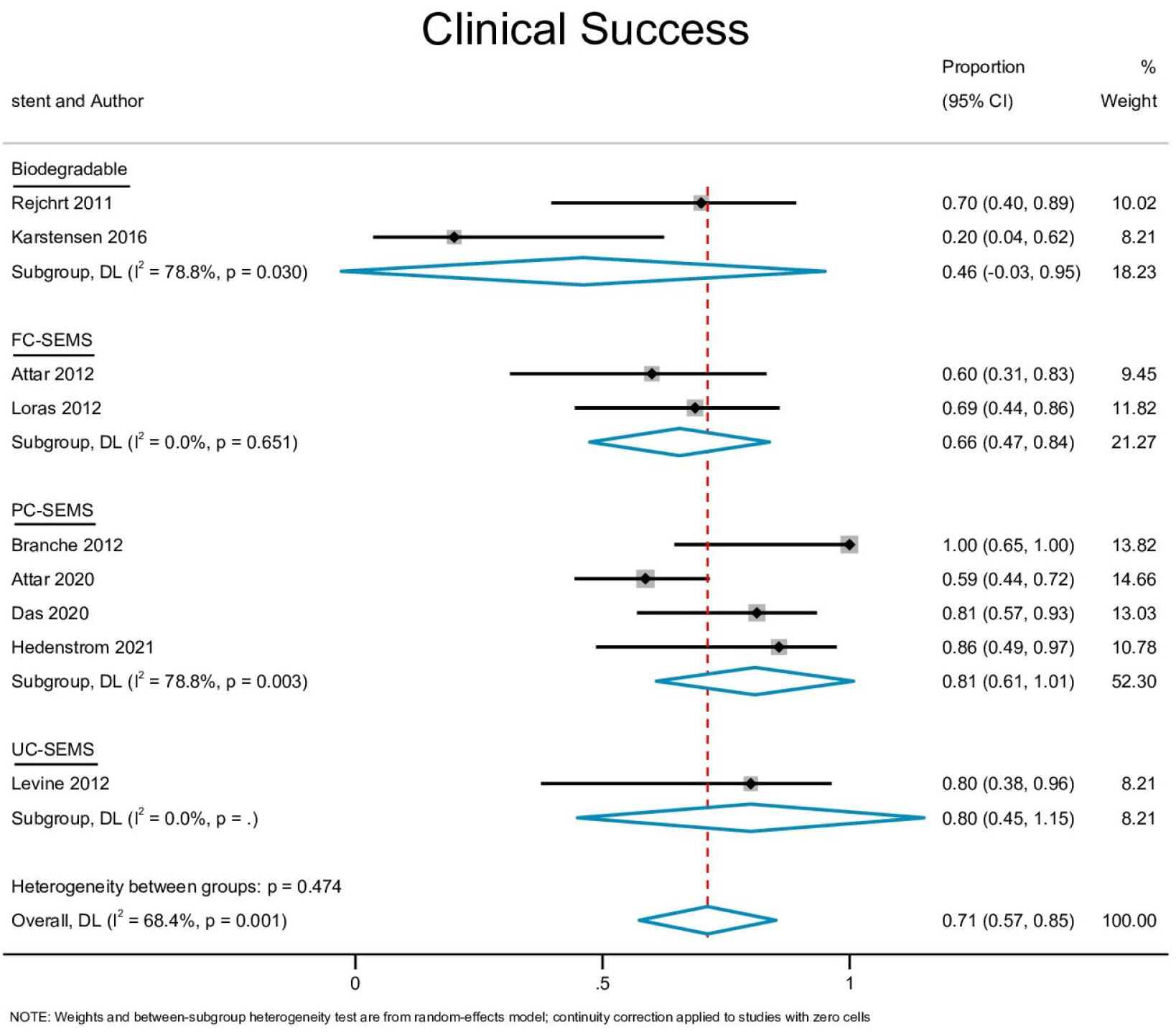
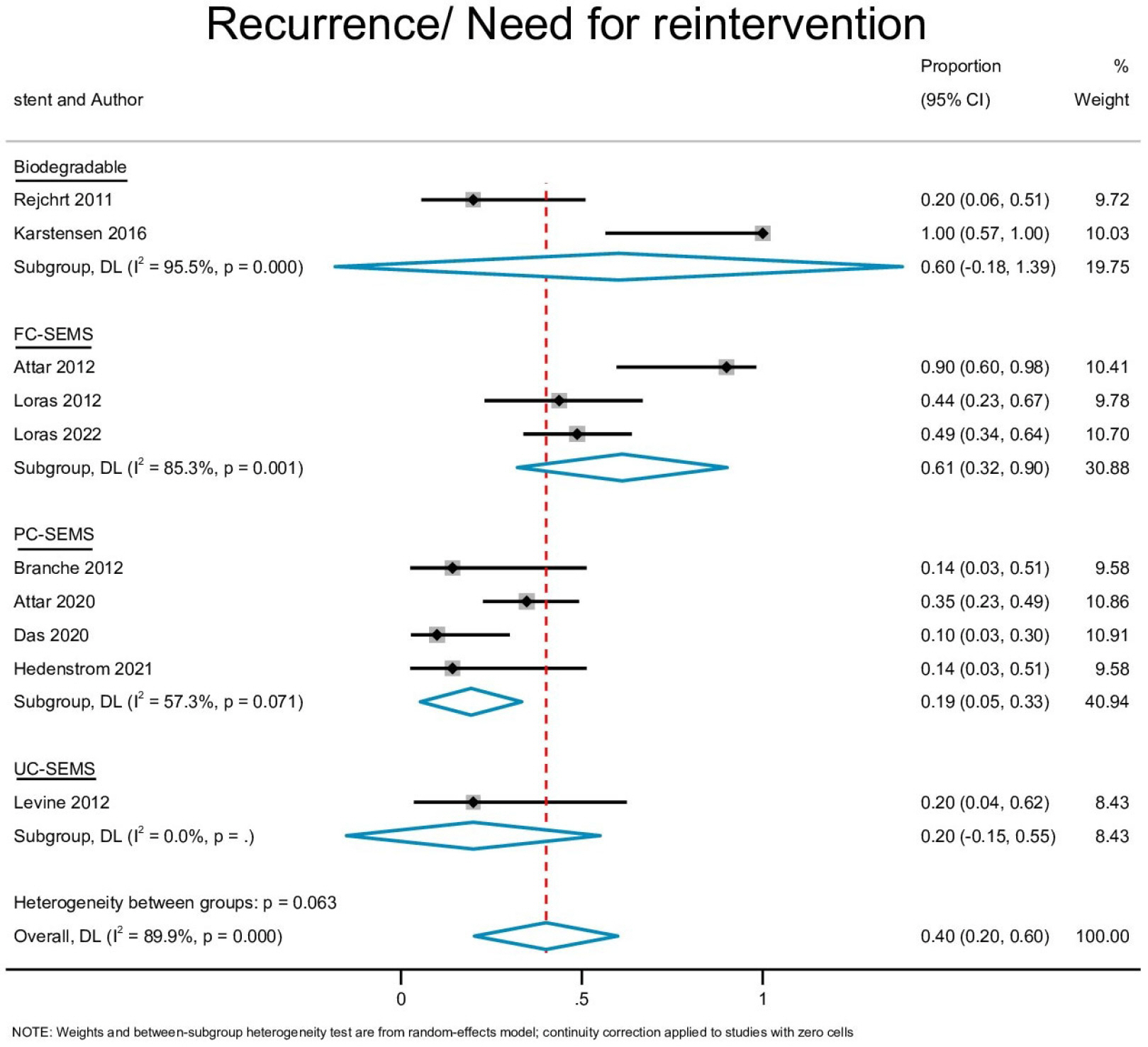
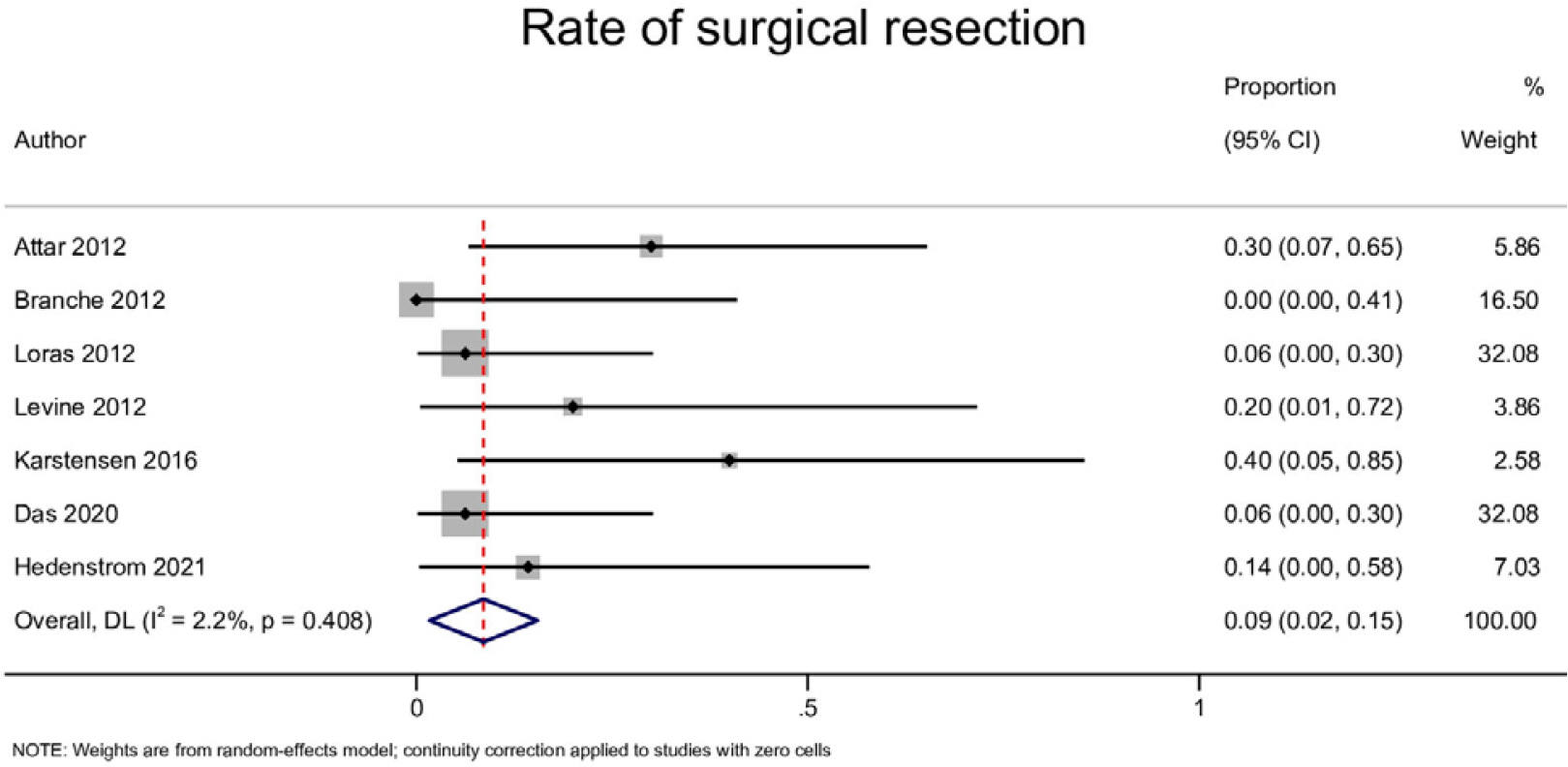
Ten studies with 170 patients were included in the present analysis. The pooled event rates for technical success, clinical success, stent migration, and post-procedural pain were 98.2% (95% CI, 95.8-100), 71.3% (95% CI, 57.4-85.1), 32% (95% CI, 0.0-65.3) and 20.2% (95% CI, 4.1-36.2), respectively. The cumulative recurrence rate and need for surgery were 40.1% (95% CI, 20.3-59.9) and 8.6% (95% CI, 1.7-15.5), respectively. Subgroup analysis showed that partially-covered (PC) self-expanding metallic stent (SEMS) was significantly better than fully-covered SEMS with a lower stent migration rate and symptom recurrence rate.
Conclusions
Overall efficacy of stents in the management of CD-related stricture remains moderate with a low complication rate. Among the stents, PC-SEMS may be associated with a more favorable outcome. Future studies will be needed to determine the long-term benefits of endoscopic stenting.
Figure
Reference
-
1. Rodríguez-Lago I, Gisbert JP. 2020; The role of immunomodulators and biologics in the medical management of stricturing Crohn's disease. J Crohns Colitis. 14:557–566. DOI: 10.1093/ecco-jcc/jjz158. PMID: 31541235.2. Solberg IC, Vatn MH, Høie O, et al. 2007; Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 5:1430–1438. DOI: 10.1016/j.cgh.2007.09.002. PMID: 18054751.3. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. 2010; The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 105:289–297. DOI: 10.1038/ajg.2009.579. PMID: 19861953.4. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. 1990; Predictability of the postoperative course of Crohn's disease. Gastroenterology. 99:956–963. DOI: 10.1016/0016-5085(90)90613-6.5. Butt WT, Ryan ÉJ, Boland MR, et al. 2020; Strictureplasty versus bowel resection for the surgical management of fibrostenotic Crohn's disease: a systematic review and meta-analysis. Int J Colorectal Dis. 35:705–717. DOI: 10.1007/s00384-020-03507-z. PMID: 32048011.6. Morar PS, Faiz O, Warusavitarne J, et al. 2015; Systematic review with meta-analysis: endoscopic balloon dilatation for Crohn's disease strictures. Aliment Pharmacol Ther. 42:1137–1148. DOI: 10.1111/apt.13388. PMID: 26358739.7. Sabaté JM, Villarejo J, Bouhnik Y, et al. 2003; Hydrostatic balloon dilatation of Crohn's strictures. Aliment Pharmacol Ther. 18:409–413. DOI: 10.1046/j.1365-2036.2003.01715.x. PMID: 12940926.8. Stroup DF, Berlin JA, Morton SC, et al. 2000; Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283:2008–2012. DOI: 10.1001/jama.283.15.2008. PMID: 10789670.9. Stang A. 2010; Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25:603–605. DOI: 10.1007/s10654-010-9491-z. PMID: 20652370.10. DerSimonian R, Laird N. 1986; Meta-analysis in clinical trials. Control Clin Trials. 7:177–188. DOI: 10.1016/0197-2456(86)90046-2.11. Mohan BP, Adler DG. 2019; Heterogeneity in systematic review and meta-analysis: how to read between the numbers. Gastrointest Endosc. 89:902–903. DOI: 10.1016/j.gie.2018.10.036. PMID: 30902218.12. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. 1991; Publication bias in clinical research. Lancet. 337:867–872. DOI: 10.1016/0140-6736(91)90201-Y.13. Rejchrt S, Kopacova M, Brozik J, Bures J. 2011; Biodegradable stents for the treatment of benign stenoses of the small and large intestines. Endoscopy. 43:911–917. DOI: 10.1055/s-0030-1256405. PMID: 21623562.14. Attar A, Maunoury V, Vahedi K, et al. 2012; Safety and efficacy of extractible self-expandable metal stents in the treatment of Crohn's disease intestinal strictures: a prospective pilot study. Inflamm Bowel Dis. 18:1849–1854. DOI: 10.1002/ibd.22844. PMID: 22161935.15. Branche J, Attar A, Vernier-Massouille G, et al. Extractible self-expandable metal stent in the treatment of Crohn's disease anastomotic strictures. Endoscopy. 2012; 44 Suppl 2 UCTN:E325–E326. DOI: 10.1055/s-0032-1309854. PMID: 23012003.16. Levine RA, Wasvary H, Kadro O. 2012; Endoprosthetic management of refractory ileocolonic anastomotic strictures after resection for Crohn's disease: report of nine-year follow-up and review of the literature. Inflamm Bowel Dis. 18:506–512. DOI: 10.1002/ibd.21739. PMID: 21542067.17. Loras C, Pérez-Roldan F, Gornals JB, et al. 2012; Endoscopic treatment with self-expanding metal stents for Crohn's disease strictures. Aliment Pharmacol Ther. 36:833–839. DOI: 10.1111/apt.12039. PMID: 22966851.18. Karstensen JG, Christensen KR, Brynskov J, Rønholt C, Vilmann P, Hendel J. 2016; Biodegradable stents for the treatment of bowel strictures in Crohn's disease: technical results and challenges. Endosc Int Open. 4:E296–E300. DOI: 10.1055/s-0042-101940. PMID: 27004247. PMCID: PMC4798842.19. Attar A, Branche J, Coron E, et al. 2021; An anti-migration self-expandable and removable metal stent for Crohn's disease strictures: a nationwide study from GETAID and SFED. J Crohns Colitis. 15:521–528. DOI: 10.1093/ecco-jcc/jjaa208. PMID: 33106876.20. Das R, Singh R, Din S, et al. 2020; Therapeutic resolution of focal, predominantly anastomotic Crohn's disease strictures using removable stents: outcomes from a single-center case series in the United Kingdom. Gastrointest Endosc. 92:344–352. DOI: 10.1016/j.gie.2020.01.053. PMID: 32081614.21. Hedenström P, Stotzer PO. 2021; Endoscopic treatment of Crohn-related strictures with a self-expandable stent compared with balloon dilation: a prospective, randomised, controlled study. BMJ Open Gastroenterol. 8:e000612. DOI: 10.1136/bmjgast-2021-000612. PMID: 33722805. PMCID: PMC7970316.22. Loras C, Andújar X, Gornals JB, et al. 2022; Self-expandable metal stents versus endoscopic balloon dilation for the treatment of strictures in Crohn's disease (ProtDilat study): an open-label, multicentre, randomised trial. Lancet Gastroenterol Hepatol. 7:332–341. DOI: 10.1016/S2468-1253(21)00386-1.23. Currie A, Christmas C, Aldean H, Mobasheri M, Bloom IT. 2014; Systematic review of self-expanding stents in the management of benign colorectal obstruction. Colorectal Dis. 16:239–245. DOI: 10.1111/codi.12389. PMID: 24033989.24. Loras Alastruey C, Andújar Murcia X, Esteve Comas M. 2016; The role of stents in the treatment of Crohn's disease strictures. Endosc Int Open. 4:E301–E308. DOI: 10.1055/s-0042-101786. PMID: 27014743. PMCID: PMC4804954.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stricturing Crohn's disease: what is the role of endoscopic stenting? A systematic review
- Update of endoscopic management of Crohn’s disease strictures
- Endoscopic Balloon Dilation for Crohn’s Disease-Associated Strictures
- Current status of endoscopic balloon dilation for Crohn's disease
- Systematic Review and Meta-analysis in Digestive Cancer Research