Cancer Res Treat.
2022 Oct;54(4):1167-1174. 10.4143/crt.2021.1040.
Adjuvant Imatinib Treatment for 5 Years versus 3 Years in Patients with Ruptured Localized Gastrointestinal Stromal Tumor: A Retrospective Analysis
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2534196
- DOI: http://doi.org/10.4143/crt.2021.1040
Abstract
- Purpose
Three years of adjuvant imatinib is the standard treatment for resected gastrointestinal stromal tumors (GISTs) with rupture, but the recurrence rate is prominently high. We aimed to investigate the efficacy and safety of 5-year adjuvant imatinib compared with 3-year treatment in patients with a ruptured GIST following surgical resection.
Materials and Methods
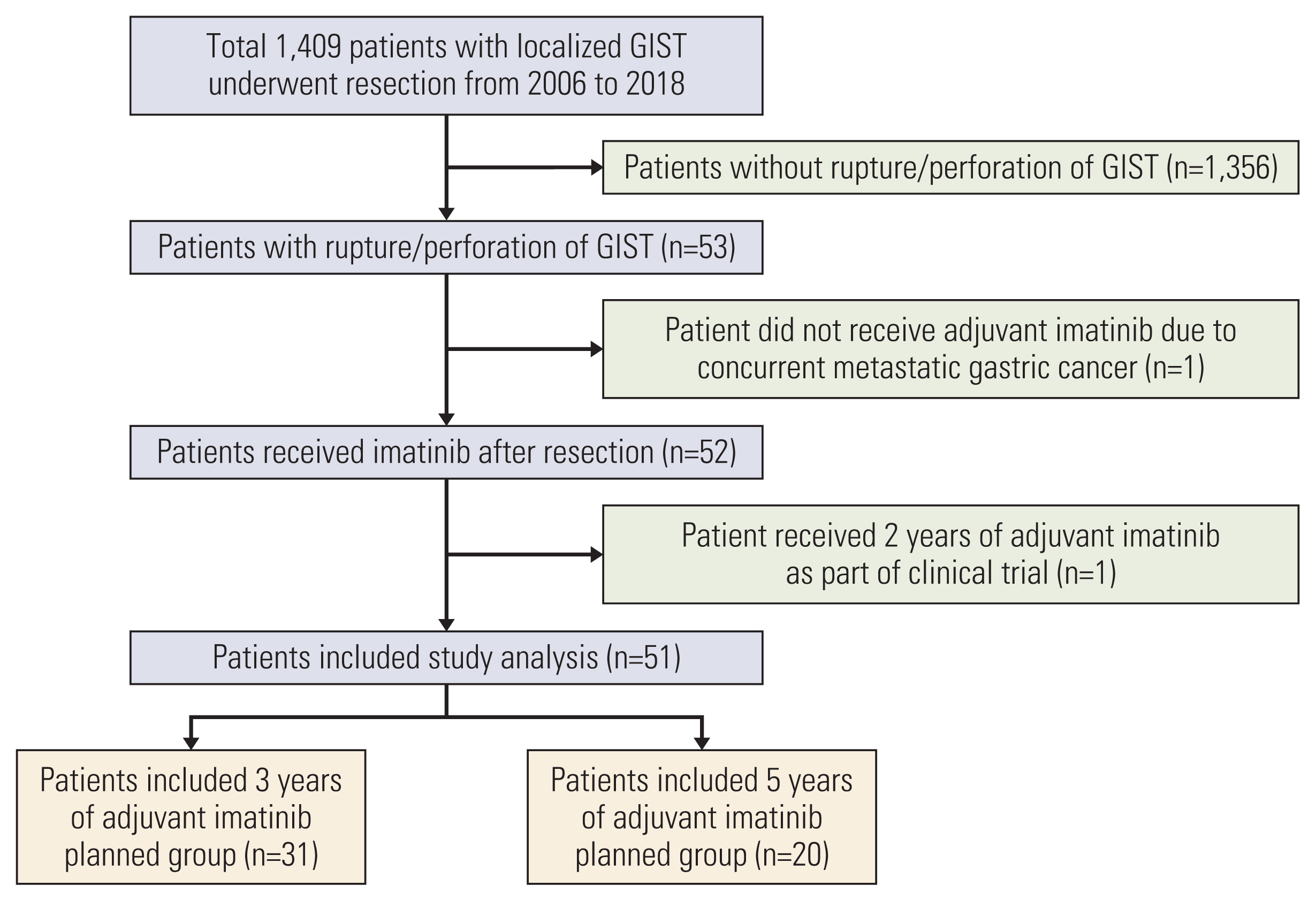
A total of 51 patients were included in the analysis. The assessment of GIST rupture was based on Nishida’s classification. Twenty patients who were diagnosed before November 2013 were treated with 5 years of imatinib, and 31 patients who were diagnosed after November 2013 were treated with 3 years of imatinib. We retrospectively compared the clinical outcomes of the two groups.
Results
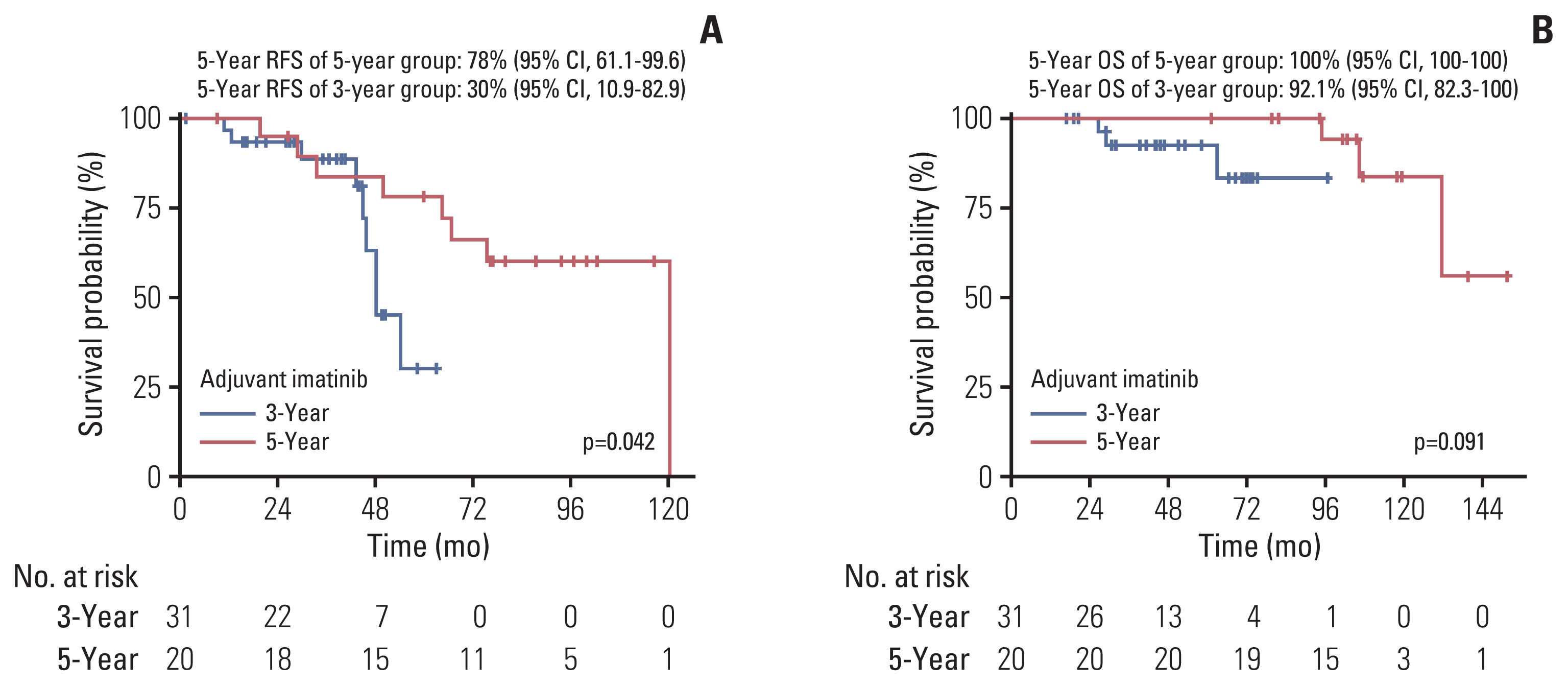
Baseline characteristics and the incidence of the adverse events were generally comparable between the two groups. During a median follow-up duration of 43.8 months and 104.2 months in the 3- and 5-year group, 8 and 9 patients had a disease recurrence, respectively. The 5-year group showed better recurrence-free survival (RFS) than the 3-year group. In multivariate analysis, low mitotic index was a significant independent favorable prognostic factor for RFS, while 5-year imatinib treatment was marginally associated with a favorable RFS.
Conclusion
Five years of adjuvant imatinib treatment in patients with ruptured GIST was associated with favorable survival outcomes with manageable toxicity profiles. Our findings warrant validation and confirmation in future studies.
Figure
Reference
-
References
1. Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021; 7:22.
Article2. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998; 279:577–80.
Article3. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–65.
Article4. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003; 299:708–10.
Article5. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29:iv68–78.6. Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour: the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011; 37:890–6.7. Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Strobel P, Wardelmann E, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010; 97:1854–9.
Article8. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–9.
Article9. Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat. 2016; 48:1155–66.
Article10. National Comprehensive Cancer Network. Gastrointestinal stromal tumor (version: 1.2021) [Internet]. Plymouth Meting, PA: National Comprehensive Cancer Network;c2021. [cited 2021 Jun 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf .11. Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schutte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012; 307:1265–72.
Article12. Zhao R, Wang Y, Huang Y, Cui Y, Xia L, Chen Y, et al. Adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: a retrospective cohort study. Sci Rep. 2017; 7:16834.
Article13. Raut CP, Espat NJ, Maki RG, Araujo DM, Trent J, Williams TF, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: the PERSIST-5 clinical trial. JAMA Oncol. 2018; 4:e184060.14. Rutkowski P, Zietek M, Cybulska-Stopa B, Streb J, Gluszek S, Jankowski M, et al. The analysis of 3-year adjuvant therapy with imatinib in patients with high-risk molecular profiled gastrointestinal stromal tumors (GIST) treated in routine practice. Eur J Surg Oncol. 2021; 47:1191–5.
Article15. Nishida T, Holmebakk T, Raut CP, Rutkowski P. Defining tumor rupture in gastrointestinal stromal tumor. Ann Surg Oncol. 2019; 26:1669–75.
Article16. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006; 130:1466–78.
Article17. Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012; 13:265–74.
Article18. Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hermes B, Schutte J, et al. Survival outcomes associated with 3 years vs 1 year of adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: an analysis of a randomized clinical trial after 10-year follow-up. JAMA Oncol. 2020; 6:1241–6.
Article19. Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016; 40:39–46.
Article20. Nishida T, Cho H, Hirota S, Masuzawa T, Chiguchi G, Tsujinaka T, et al. Clinicopathological features and prognosis of primary GISTs with tumor rupture in the real world. Ann Surg Oncol. 2018; 25:1961–9.
Article21. Joensuu H, Wardelmann E, Sihto H, Eriksson M, Sundby Hall K, Reichardt A, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trial. JAMA Oncol. 2017; 3:602–9.
Article22. Kim EJ, Ryu MH, Park SR, Beck MY, Lee WJ, Lee MW, et al. Systemic steroid treatment for imatinib-associated severe skin rash in patients with gastrointestinal stromal tumor: a phase II study. Oncologist. 2020; 25:e1785–93.
Article23. Kim JH, Ryu MH, Yoo C, Chae H, Na H, Beck M, et al. Long-term survival outcome with tyrosine kinase inhibitors and surgical intervention in patients with metastatic or recurrent gastrointestinal stromal tumors: a 14-year, single-center experience. Cancer Med. 2019; 8:1034–43.
Article24. Blay JY, Rutkowski P. Adherence to imatinib therapy in patients with gastrointestinal stromal tumors. Cancer Treat Rev. 2014; 40:242–7.
Article25. Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Wozniak A, Limon J, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007; 14:2018–27.
Article26. Casali PG, Le Cesne A, Poveda Velasco A, Kotasek D, Rutkowski P, Hohenberger P, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group intergroup randomized trial in collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015; 33:4276–83.
Article27. Holmebakk T, Bjerkehagen B, Boye K, Bruland O, Stoldt S, Sundby Hall K. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016; 103:684–91.
Article28. Holmebakk T, Hompland I, Bjerkehagen B, Stoldt S, Bruland OS, Hall KS, et al. Recurrence-free survival after resection of gastric gastrointestinal stromal tumors classified according to a strict definition of tumor rupture: a population-based study. Ann Surg Oncol. 2018; 25:1133–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imatinib-induced DRESS Syndrome in Gastrointestinal Stromal Tumor
- Successful Endoscopic Resection of a Rectal Gastrointestinal Stromal Tumor Larger Than 5 cm
- A High Risk Group in the Modified National Institutes of Health Consensus Criteria for the Gastrointestinal Stromal Tumor: A Clear Indication of the Adjuvant Imatinib
- Diagnosis and Treatment of Imatinib-induced Pneumonitis in a Patient with High-risk Rectal Gastrointestinal Stromal Tumor
- A Case of Generalized Keratosis Pilaris Induced by Imatinib Mesylate