Cancer Res Treat.
2022 Oct;54(4):1038-1052. 10.4143/crt.2021.698.
Clinical Evidence of Chemotherapy or Endocrine Therapy Maintenance in Patients with Metastatic Breast Cancer: Meta-Analysis of Randomized Clinical Trials and Propensity Score Matching of Multicenter Cohort Study
- Affiliations
-
- 1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Department of Medical Oncology, Breast Tumor Centre, Phase I Clinical Trial Centre, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 2Division of Science and Technology, Beijing Normal University-Hong Kong Baptist University United International College, Hong Kong Baptist University, Zhuhai, China
- 3Meizhou Academy of Medical Science, Meizhou Hospital Affiliated of Sun Yat-sen University Medical University, Meizhou, China
- 4Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 5First People’s Hospital of Foshan, Foshan Affiliated Hospital of Sun Yat-sen University, Foshan, China
- 6State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- KMID: 2534183
- DOI: http://doi.org/10.4143/crt.2021.698
Abstract
- Purpose
This study aims to comprehensively evaluate the clinical efficacy of chemotherapy or endocrine therapy maintenance in metastatic breast cancer (MBC) patients.
Materials and Methods
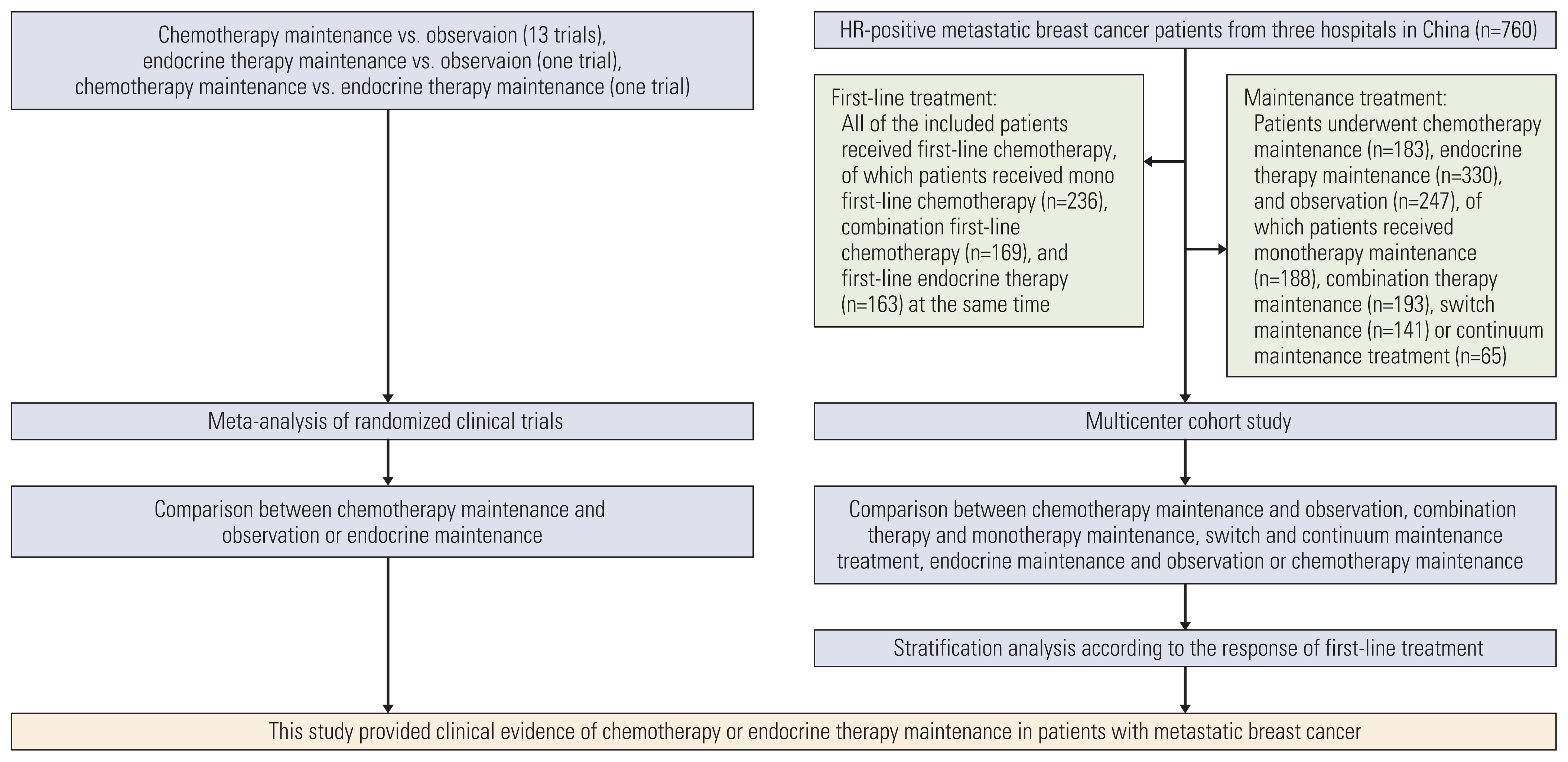
The meta-analysis of randomized clinical trials (RCTs) and propensity score matching of multicenter cohort study evaluated MBC patients who underwent first-line chemotherapy or endocrine therapy maintenance. This study is registered with PROSPERO: CRD42017071858 and ClinicalTrials.gov: NCT04258163.
Results
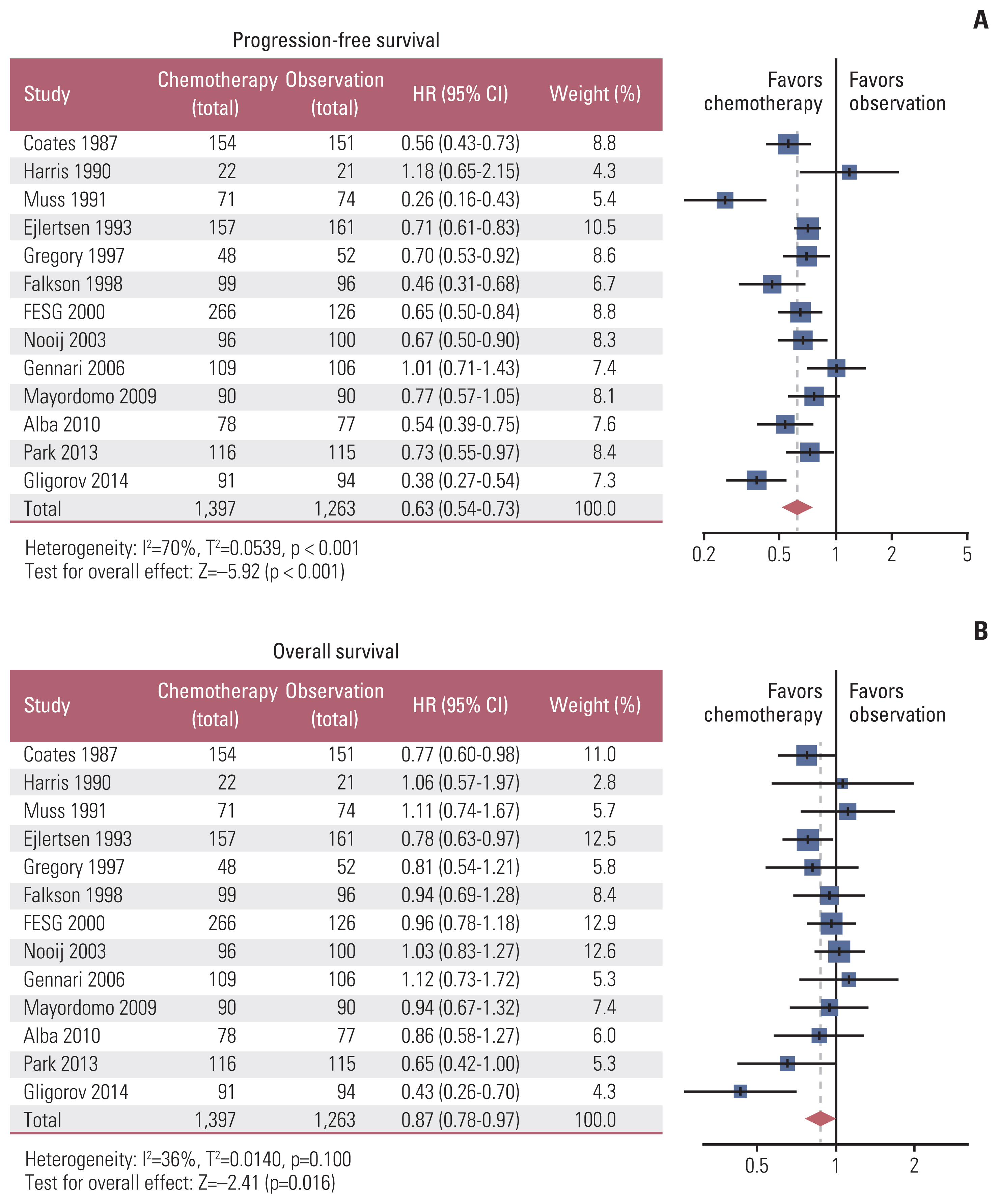
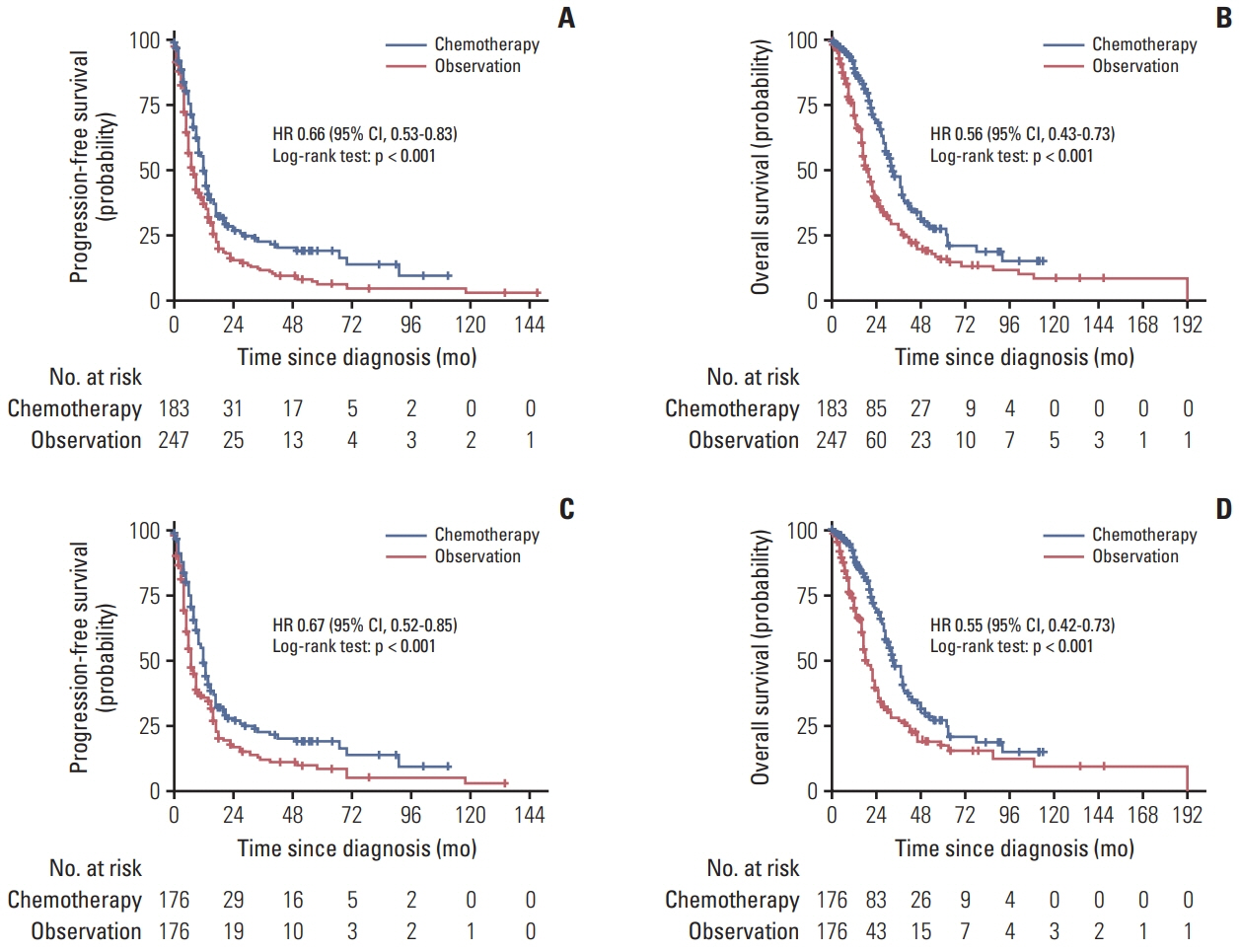
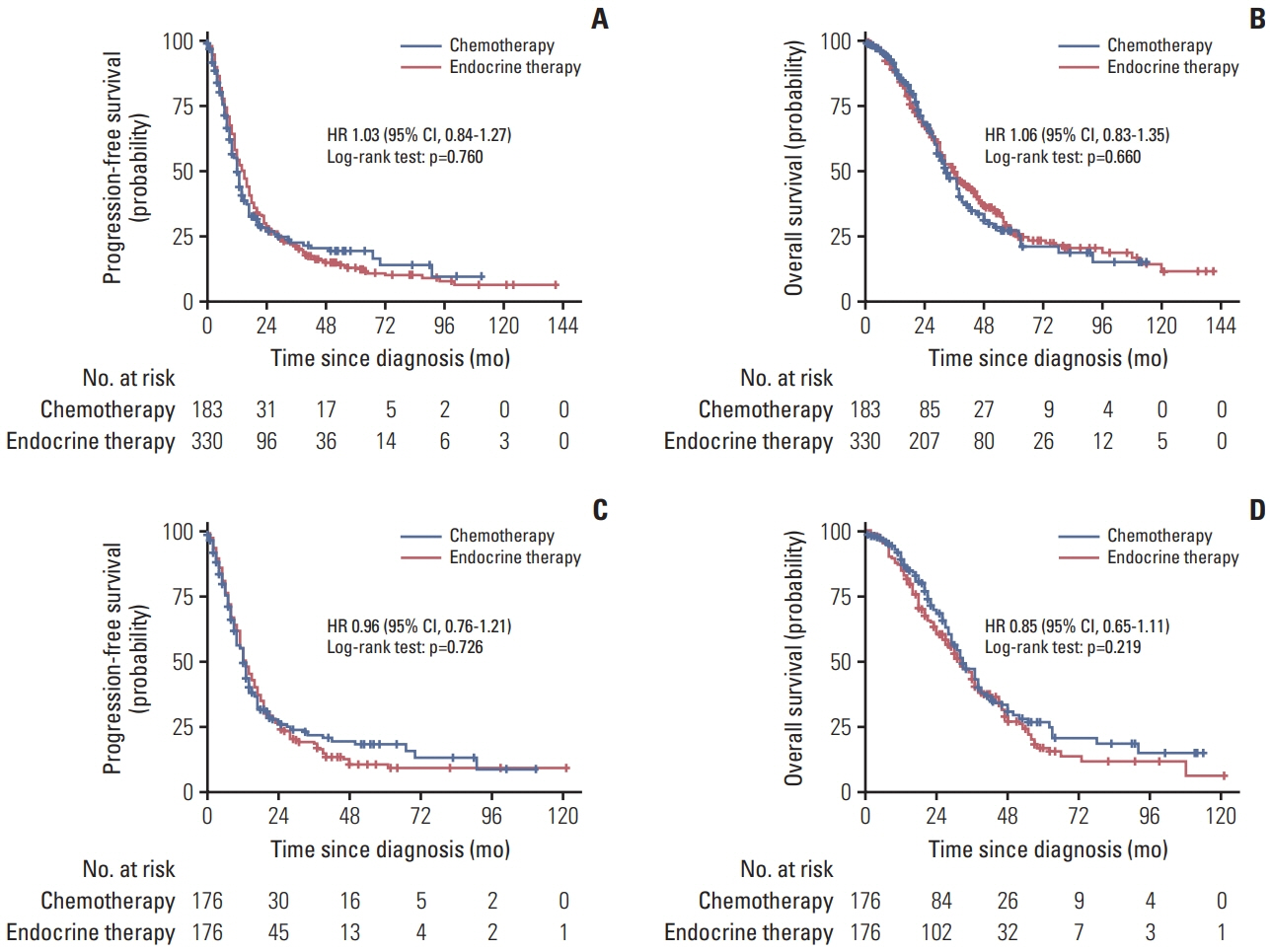
A total of 2,867 patients from 15 RCTs and 760 patients from multicenter cohort were included. The results from meta-analysis showed that chemotherapy maintenance improved progression-free survival (PFS) (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.54 to 0.73; p < 0.001; moderate-quality evidence) and overall survival (OS) (HR, 0.87; 95% CI 0.78 to 0.97; p=0.016; high-quality evidence) than observation. In the cohort study, for hormone receptor–positive MBC patients, chemotherapy maintenance improved PFS (HR, 0.67; 95% CI, 0.52 to 0.85; p < 0.001) and OS (HR, 0.55; 95% CI 0.42 to 0.73; p < 0.001) compared with observation, and endocrine therapy maintenance also improved PFS (HR, 0.65; 95% CI, 0.53 to 0.80; p < 0.001) and OS (HR, 0.55; 95% CI, 0.44 to 0.69; p < 0.001). There were no differences between chemotherapy and endocrine therapy maintenance in PFS and OS (all p > 0.05). Regardless of the continuum or switch maintenance therapy, showed prolonged survival in MBC patients who were response to first-line treatment.
Conclusion
This study provided evidences for survival benefits of chemotherapy and endocrine therapy maintenance in MBC patients, and there was no difference efficacy between chemotherapy and endocrine therapy maintenance for hormone receptor–positive patients.
Keyword
Figure
Reference
-
References
1. Gregory RK, Powles TJ, Chang JC, Ashley S. A randomised trial of six versus twelve courses of chemotherapy in metastatic carcinoma of the breast. Eur J Cancer. 1997; 33:2194–7.2. Muss HB, Case LD, Richards F 2nd, White DR, Cooper MR, Cruz JM, et al. Interrupted versus continuous chemotherapy in patients with metastatic breast cancer. The Piedmont Oncology Association. N Engl J Med. 1991; 325:1342–8.3. Park YH, Jung KH, Im SA, Sohn JH, Ro J, Ahn JH, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol. 2013; 31:1732–9.4. Ejlertsen B, Pfeiffer P, Pedersen D, Mouridsen HT, Rose C, Overgaard M, et al. Decreased efficacy of cyclophosphamide, epirubicin and 5-fluorouracil in metastatic breast cancer when reducing treatment duration from 18 to 6 months. Eur J Cancer. 1993; 29A:527–31.5. Tredan O, Follana P, Moullet I, Cropet C, Trager-Maury S, Dauba J, et al. A phase III trial of exemestane plus bevacizumab maintenance therapy in patients with metastatic breast cancer after first-line taxane and bevacizumab: a GINECO group study. Ann Oncol. 2016; 27:1020–9.6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–94.7. Higgins JP, Green S. Cochrane handbook for systematic reviews of iterventions, version 5.1. 0. Chichester: John Wiley & Sons Ltd;2011.8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.9. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.11. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–6.12. Westreich D, Lessler J, Funk MJ. Propensity score estimation: neural networks, support vector machines, decision trees (CART), and meta-classifiers as alternatives to logistic regression. J Clin Epidemiol. 2010; 63:826–33.13. Alba E, Ruiz-Borrego M, Margeli M, Rodriguez-Lescure A, Sanchez-Rovira P, Ruiz A, et al. Maintenance treatment with pegylated liposomal doxorubicin versus observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res Treat. 2010; 122:169–76.14. Coates A, Gebski V, Bishop JF, Jeal PN, Woods RL, Snyder R, et al. Improving the quality of life during chemotherapy for advanced breast cancer: a comparison of intermittent and continuous treatment strategies. N Engl J Med. 1987; 317:1490–5.15. Falkson G, Gelman RS, Pandya KJ, Osborne CK, Tormey D, Cummings FJ, et al. Eastern Cooperative Oncology Group randomized trials of observation versus maintenance therapy for patients with metastatic breast cancer in complete remission following induction treatment. J Clin Oncol. 1998; 16:1669–76.16. French Epirubicin Study Group. Epirubicin-based chemotherapy in metastatic breast cancer patients: role of dose-intensity and duration of treatment. J Clin Oncol. 2000; 18:3115–24.17. Gennari A, Amadori D, De Lena M, Nanni O, Bruzzi P, Lorusso V, et al. Lack of benefit of maintenance paclitaxel in first-line chemotherapy in metastatic breast cancer. J Clin Oncol. 2006; 24:3912–8.18. Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15:1351–60.19. Harris AL, Cantwell BM, Carmichael J, Wilson R, Farndon J, Dawes P, et al. Comparison of short-term and continuous chemotherapy (mitozantrone) for advanced breast cancer. Lancet. 1990; 335:186–90.20. Mayordomo JI, Baena JM, Cirera L, Sanchez-Rovira P, Godes MJ, Galan A, et al. Final results of a randomized trial on the role of maintenance chemotherapy with weekly paclitaxel for patients with metastatic breast cancer. J Clin Oncol. 2009; 15(Suppl):1001.21. Kloke O, Klaassen U, Oberhoff C, Hartwich G, Szanto J, Wolf E, et al. Maintenance treatment with medroxyprogesterone acetate in patients with advanced breast cancer responding to chemotherapy: results of a randomized trial. Essen Breast Cancer Study Group. Breast Cancer Res Treat. 1999; 55:51–9.22. Nooij MA, de Haes JC, Beex LV, Wildiers J, Klijn J, Becquart D, et al. Continuing chemotherapy or not after the induction treatment in advanced breast cancer patients. clinical outcomes and oncologists’ preferences. Eur J Cancer. 2003; 39:614–21.23. Gennari A, Stockler M, Puntoni M, Sormani M, Nanni O, Amadori D, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol. 2011; 29:2144–9.24. Yu YF, Wang Y, Fu TP, Chen K, Liu JQ, Yao HR. Trastuzumab combined with doublet or single-agent chemotherapy as first-line therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2018; 168:337–48.25. Xie N, Qin T, Ren W, Yao H, Yu Y, Hong H. Efficacy and safety of cyclin-dependent kinases 4 and 6 inhibitors in HR+/HER2− advanced breast cancer. Cancer Manag Res. 2020; 12:4241–50.26. Jacquet E, Lardy-Cleaud A, Pistilli B, Franck S, Cottu P, Delaloge S, et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor-positive HER2-negative metastatic breast cancer patients. Eur J Cancer. 2018; 95:93–101.27. Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer. 2016; 69:216–22.28. Kwan TT, Bardia A, Spring LM, Giobbie-Hurder A, Kalinich M, Dubash T, et al. A digital RNA signature of circulating tumor cells predicting early therapeutic response in localized and metastatic breast cancer. Cancer Discov. 2018; 8:1286–99.29. Yu Y, Zhang W, Li A, Chen Y, Ou Q, He Z, et al. Association of long noncoding RNA biomarkers with clinical immune subtype and prediction of immunotherapy response in patients with cancer. JAMA Netw Open. 2020; 3:e202149.30. Zhao J, Wang Y, Lao Z, Liang S, Hou J, Yu Y, et al. Prognostic immune-related gene models for breast cancer: a pooled analysis. Onco Targets Ther. 2017; 10:4423–33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immediate Breast Reconstruction Does Not Have a Clinically Significant Impact on Adjuvant Treatment Delay and Subsequent Survival Outcomes
- Efficacy and safety of cisplatin combined with paclitaxel concurrent radiotherapy in patients with locally advanced cervical squamous cell carcinoma
- Adjuvant Chemotherapy for Breast Cancer
- Role of Ovarian Function Suppression in Premenopausal Women with Early Breast Cancer
- The evidence for adjuvant taxanes in early breast cancer