Obstet Gynecol Sci.
2022 Sep;65(5):406-419. 10.5468/ogs.22115.
Prophylactic tranexamic acid to reduce blood loss and related morbidities during hysterectomy: a systematic review and meta-analysis of randomized controlled trials
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Alfaisal University, Riyadh, Saudi Arabia
- 2Department of Pharmacology, College of Graduate Health Sciences, University of Tennessee Healt Science Center, Memphis, TN, USA
- 3Department of Obstetrics and Gynecology, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 4Department of Obstetrics and Gynecology, Maternity and Children Hospital, Makkah, Saudi Arabia
- 5Department of Obstetrics and Gynecology, Maternity and Children Hospital, AlKharj, Saudi Arabia
- 6Department of Obstetrics and Gynecology, College of Medicine, Jeddah University, Jeddah, Saudi Arabia
- 7Department of Obstetrics and Gynecology, Prince Mohammed Bin Abdulaziz National Guard Hospital, Madinah, Saudi Arabia
- 8Department of Obstetrics and Gynecology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
- 9Department of Obstetrics and Gynecology, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 10Department of Obstetrics and Gynecology, King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia
- 11Department of Obstetrics and Gynecology, Farwaniya Hospital, Farwaniya, Kuwait, Saudi Arabia
- 12Department of Obstetrics and Gynecology, Riyadh Second Health Cluster, Riyadh, Saudi Arabia
- KMID: 2533814
- DOI: http://doi.org/10.5468/ogs.22115
Abstract
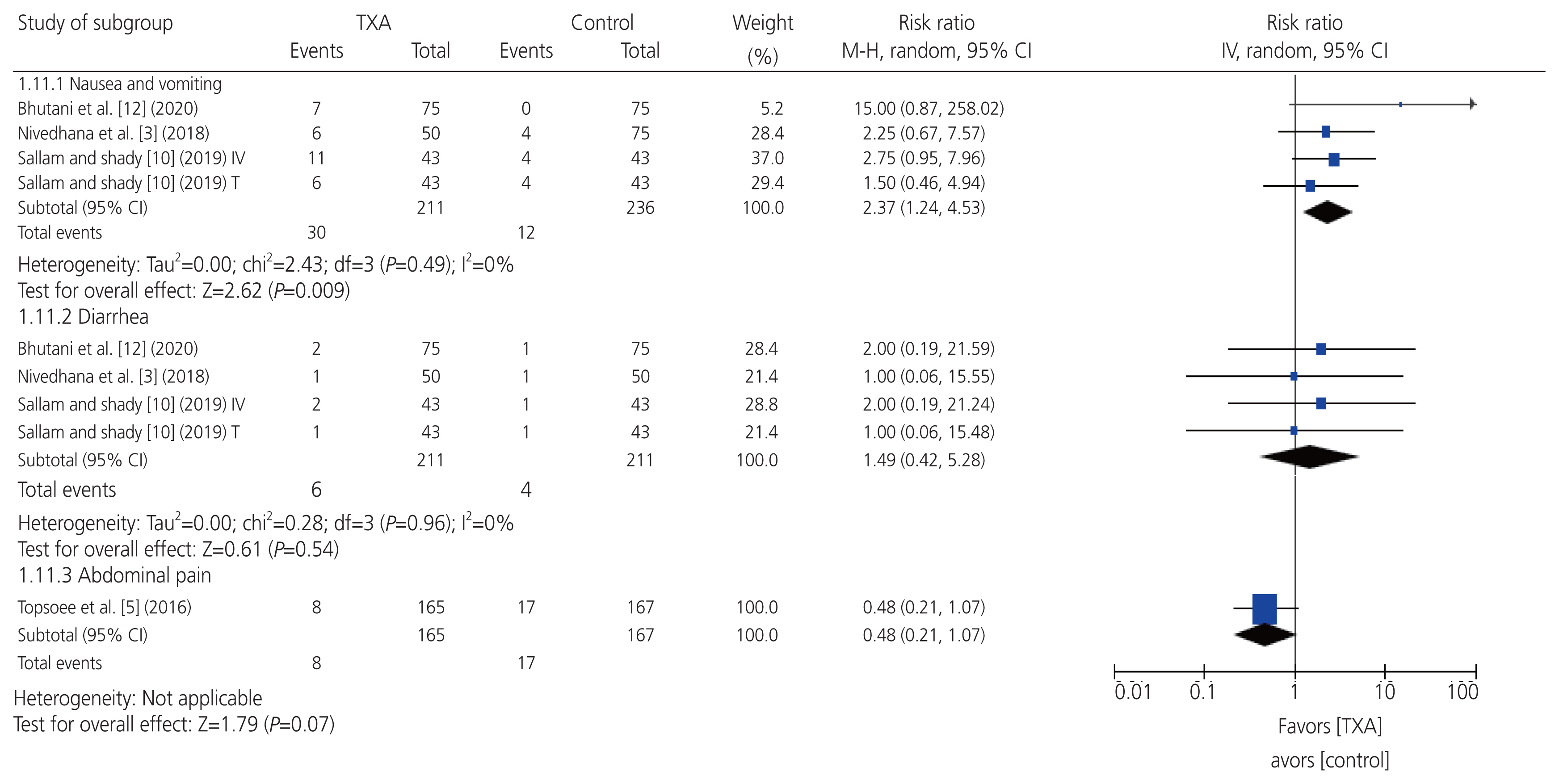
- To perform a systematic review and meta-analysis of all randomized controlled trials (RCTs) that evaluated the efficacy and safety of prophylactic tranexamic acid (TXA) versus a control (placebo or no treatment) during hysterectomy for benign conditions. Six databases were screened from inception to January 23, 2022. Eligible studies were assessed for risk of bias. Outcomes were summarized as weighted mean differences and risk ratios with 95% confidence intervals in a random-effects model. Five studies, comprising six arms and 911 patients were included in the study. Two and three studies had an overall unclear and low risk of bias, respectively. Estimated intraoperative blood loss, requirement for postoperative blood transfusion, and requirement for intraoperative topical hemostatic agents were significantly reduced in a prophylactic TXA group when compared with a control group. Moreover, postoperative hemoglobin level was significantly higher in the prophylactic TXA group than in the control group. Conversely, the frequency of self-limiting nausea and vomiting was significantly higher in the prophylactic TXA group than in the control group. There were no significant differences between the groups in terms of surgery duration, hospital stay, and diarrhea rate. All the RCTs reported no incidence of major adverse events in either group, such as mortality, thromboembolic events, visual disturbances, or seizures. There was no publication bias for any outcome, and leave-one-out sensitivity analyses demonstrated stability of the findings. Among patients who underwent hysterectomy for benign conditions, prophylactic TXA appeared largely safe and correlated with substantial reductions in estimated intraoperative blood loss and related morbidities.
Keyword
Figure
Reference
-
References
1. Khazaei Z, Goodarzi E, Sohrabivafa M, Naemi H, Mansori K. Association between the incidence and mortality rates for corpus uteri cancer and human development index (HDI): a global ecological study. Obstet Gynecol Sci. 2020; 63:141–9.
Article2. Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet Gynecol. 2013; 121:654–73.
Article3. Nivedhana AP, Jalakandan B, Gunaseelan S. Effect of prophylactic tranexamic acid on blood conservation in Indian women undergoing abdominal hysterectomy. Int J Reprod Contracept Obstet Gynecol. 2018; 7:3538–46.
Article4. Hansen CT, Møller C, Daugbjerg S, Utzon J, Kehlet H, Ottesen B. Establishment of a national Danish hysterectomy database: preliminary report on the first 13,425 hysterectomies. Acta Obstet Gynecol Scand. 2008; 87:546–57.
Article5. Topsoee MF, Bergholt T, Ravn P, Schouenborg L, Moeller C, Ottesen B, et al. Anti-hemorrhagic effect of prophylactic tranexamic acid in benign hysterectomy-a double-blinded randomized placebo-controlled trial. Am J Obstet Gynecol. 2016; 215:72.e1–8.
Article6. Koh MB, Hunt BJ. The management of perioperative bleeding. Blood Rev. 2003; 17:179–85.
Article7. Cai J, Ribkoff J, Olson S, Raghunathan V, Al-Samkari H, DeLoughery TG, et al. The many roles of tranexamic acid: an overview of the clinical indications for TXA in medical and surgical patients. Eur J Haematol. 2020; 104:79–87.
Article8. Wong J, George RB, Hanley CM, Saliba C, Yee DA, Jerath A. Tranexamic acid: current use in obstetrics, major orthopedic, and trauma surgery. Can J Anaesth. 2021; 68:894–917.
Article9. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999; 57:1005–32.10. Sallam HF, Shady NW. Reducing blood loss during abdominal hysterectomy with intravenous versus topical tranexamic acid: a double-blind randomized controlled trial. J Obstet Gynaecol India. 2019; 69:173–9.
Article11. Singh D, Bindal J. The effect of prophylactic use of intravenous tranexamic acid in hysterectomy of benign diseases. Int J Clin Obstet Gynaecol. 2020; 4:93–7.
Article12. Bhutani S, Malik R, Duhan N. Role of intravenous tranexamic acid (TXA) in reducing perioperative blood loss in hysterectomy for benign gynecological conditions. Authorea. 2020. Sep. 11. [Epub]. https://doi.org/10.22541/au.159986276.65428056 .
Article13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
Article14. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated march 2011) [Internet]. London: Cochrane;c2011. [cited 2022 Apr 2]. Available from: www.handbook.cochrane.org .15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.
Article17. Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992; 14:154–76.
Article18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
Article19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.
Article20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–101.
Article21. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135.
Article22. Abu-Zaid A, Altowairqi AK, Dissanayaka T, Oganesyan A, Bhagavathul AS, Alhabeeb H, et al. A systematic review and dose-response meta-analysis on the efficacy of dapagliflozin in patients with type 1 diabetes mellitus. Pharmacol Res. 2021; 165:105456.
Article23. Abu-Zaid A, Magzoub D, Aldehami MA, Behiry AA, Bhagavathula AS, Hajji R. The effect of iron supplementation on FGF23 in chronic kidney disease patients: a systematic review and time-response meta-analysis. Biol Trace Elem Res. 2021; 199:4516–24.
Article24. Traylor J, Runge M, Friedman J, Nixon KE, Tsai SC, Chaudhari A, et al. Intravenous tranexamic acid prior to hysterectomy for reduction of intraoperative blood loss: a randomized placebo-controlled trial. J Minim Invasive Gynecol. 2020; 27:S5.
Article25. Prasad R, Patki A, Padhy S, Ramchandran G. Single intravenous bolus versus perioperative continuous infusion of tranexamic acid to reduce blood loss in abdominal oncosurgical procedures: a prospective randomized double-blind clinical study. J Anaesthesiol Clin Pharmacol. 2018; 34:529–34.
Article26. Gerdessen L, Meybohm P, Choorapoikayil S, Herrmann E, Taeuber I, Neef V, et al. Comparison of common perioperative blood loss estimation techniques: a systematic review and meta-analysis. J Clin Monit Comput. 2021; 35:245–58.
Article27. Thompson JD, Birch HW. Indications of hysterectomy. Clin Obstet Gynecol. 1981; 24:1245–58.28. Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007; 110:1301–3.29. Chen CL, Xu YJ, Liu P, Zhu JH, Ma B, Zeng BL, et al. Characteristics of vascular supply to uterine leiomyoma: an analysis of digital subtraction angiography imaging in 518 cases. Eur Radiol. 2013; 23:774–9.
Article30. Dockeray CJ, Sheppard BL, Daly L, Bonnar J. The fibrinolytic enzyme system in normal menstruation and excessive uterine bleeding and the effect of tranexamic acid. Eur J Obstet Gynecol Reprod Biol. 1987; 24:309–18.
Article31. Gleeson NC. Cyclic changes in endometrial tissue plasminogen activator and plasminogen activator inhibitor type 1 in women with normal menstruation and essential menorrhagia. Am J Obstet Gynecol. 1994; 171:178–83.
Article32. Levy JH, Tanaka KA. Management of surgical hemostasis: systemic agents. Vascular. 2008; 16(Suppl 1):S14–21.33. Sileshi B, Achneck HE, Lawson JH. Management of surgical hemostasis: topical agents. Vascular. 2008; 16(Suppl 1):S22–8.34. Fraser IS, Porte RJ, Kouides PA, Lukes AS. A benefit-risk review of systemic haemostatic agents: part 1: in major surgery. Drug Saf. 2008; 31:217–30.35. Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs. 1985; 29:236–61.
Article36. Bellos I, Pergialiotis V. Tranexamic acid for the prevention of postpartum hemorrhage in women undergoing cesarean delivery: an updated meta-analysis. Am J Obstet Gynecol. 2022; 226:510–23e22.
Article37. Koh A, Adiamah A, Gomez D, Sanyal S. Safety and efficacy of tranexamic acid to minimise perioperative bleeding in hepatic surgery: a systematic review and meta-analysis. World J Surg. 2022; 46:441–49.
Article38. Chan CC, Chan YY, Tanweer F. Systematic review and meta-analysis of the use of tranexamic acid in tonsillectomy. Eur Arch Otorhinolaryngol. 2013; 270:735–48.
Article39. Hu M, Liu ZB, Bi G. Efficacy and safety of tranexamic acid in orthopaedic trauma surgery: a meta-analysis. Eur Rev Med Pharmacol Sci. 2019; 23:11025–31.40. Kim DH, Kim S, Kang H, Jin HJ, Hwang SH. Efficacy of tranexamic acid on operative bleeding in endoscopic sinus surgery: a meta-analysis and systematic review. Laryngoscope. 2019; 129:800–7.
Article41. Ma QM, Han GS, Li BW, Li XJ, Jiang T. Effectiveness and safety of the use of antifibrinolytic agents in total-knee arthroplasty: a meta-analysis. Medicine (Baltimore). 2020; 99:e20214.42. Murji A, Lam M, Allen B, Richard L, Shariff SZ, Austin PC, et al. Risks of preoperative anemia in women undergoing elective hysterectomy and myomectomy. Am J Obstet Gynecol. 2019; 221:629e1–629.e18.
Article43. Rock WA Jr, Meeks GR. Managing anemia and blood loss in elective gynecologic surgery patients. J Reprod Med. 2001; 46:507–14.44. Rawn J. The silent risks of blood transfusion. Curr Opin Anaesthesiol. 2008; 21:664–8.
Article45. Jain SB, Shikha . Decline in blood loss with use of tranexamic acid in cases of hysterectomy: a retrospective observational study in a teaching hospital of central India. IJOGR. 2021; 8:26–30.
Article46. Taeuber I, Weibel S, Herrmann E, Neef V, Schlesinger T, Kranke P, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis, and meta-regression. JAMA Surg. 2021; 156:e210884.47. Xu JW, Qiang H, Li TL, Wang Y, Wei XX, Li F. Efficacy of topical vs intravenous tranexamic acid in reducing blood loss and promoting wound healing in bone surgery: a systematic review and meta-analysis. World J Clin Cases. 2021; 9:4210–20.48. Teoh WY, Tan TG, Ng KT, Ong KX, Chan XL, Hung Tsan SE, et al. Prophylactic topical tranexamic acid versus placebo in surgical patients: a systematic review and meta-analysis*. Ann Surg. 2021; 273:676–83.49. Hafidh B, Latifah HM, Gari A, Alshahrani MS, AlSghan R, Alkhamis WH, et al. Vasopressin to control blood loss during hysterectomy: a systematic review and meta-analysis of randomized controlled trials. J Minim Invasive Gynecol. 2022; 29:355–694e2.
Article50. Sun Q, Li J, Chen J, Zheng C, Liu C, Jia Y. Comparison of intravenous, topical or combined routes of tranexamic acid administration in patients undergoing total knee and hip arthroplasty: a meta-analysis of randomised controlled trials. BMJ Open. 2019; 9:e024350.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of intravenous tranexamic acid on perioperative bleeding and transfusion in spine surgery: systematic review and meta-analysis of randomized controlled trials
- Introduction to systematic review and meta-analysis
- Minimal Dose of Tranexamic Acid Is Effective in Reducing Blood Loss in Complex Spine Surgeries: A Randomized Double-Blind Placebo Controlled Study
- Effect of Tranexamic Acid on Blood Loss and Blood Transfusion Reduction after Total Knee Arthroplasty
- Tranexamic acid - a promising hemostatic agent with limitations: a narrative review