Clin Endosc.
2022 Sep;55(5):674-682. 10.5946/ce.2021.210.
Efficacy of a novel channel-cleaning ball brush for endoscope reprocessing: a randomized controlled trial
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Uijeongbu Eulji Medical Center, Eulji University School of Medicine, Uijeongbu, Korea
- 2Department of Laboratory Medicine, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Uijeongbu Eulji Medical Center, Eulji University School of Medicine, Uijeongbu, Korea
- KMID: 2533307
- DOI: http://doi.org/10.5946/ce.2021.210
Abstract
- Background/Aims
Endoscopic channels are difficult to clean and can cause infection transmission. We examined the effectiveness of a newly developed channel-cleaning ball brush (BB), which is sucked into the endoscopic channel and scrapes and cleans the lumen as it passes through.
Methods
The upper and lower gastrointestinal endoscopes used for patient examinations were randomly selected as the conventional brush (CB) or BB group. After manual cleaning, the presence or absence of carbohydrates, proteins, adenosine triphosphate, and hemoglobin was assessed.
Results
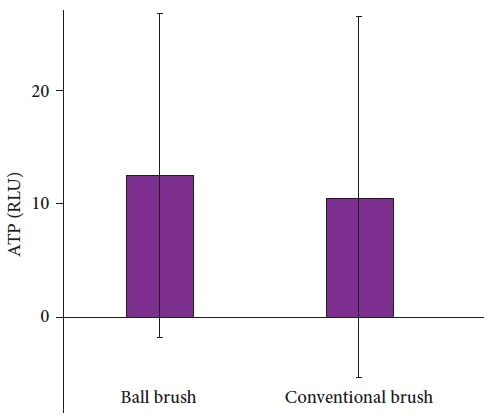
Fifty-six and 58 endoscopes were cleaned with the CB and BB, respectively. Carbohydrate and protein were detected in one (1.8%) and two endoscopes (3.4%) in the CB and BB groups, respectively (p=1.000). Hemoglobin was observed in one (1.8%) and three endoscopes (5.2%) in the CB and BB groups, respectively (p=0.636). The adenosine triphosphate levels were 10.6±15.9 and 12.5±14.3 relative light units in the CB and BB groups, respectively (p=0.496). Twenty-seven (48.2%) and 19 (32.8%) endoscopes were positive for microbial cultures in the CB and BB groups, respectively (p=0.136).
Conclusions
The efficacy of BB was not significantly different from that of CB in the endoscopic channel-cleaning process.
Figure
Reference
-
1. Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014; 312:1447–1455.2. Kovaleva J, Peters FT, van der Mei HC, et al. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev. 2013; 26:231–254.3. Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993; 118:117–128.4. McCafferty CE, Aghajani MJ, Abi-Hanna D, et al. An update on gastrointestinal endoscopy-associated infections and their contributing factors. Ann Clin Microbiol Antimicrob. 2018; 17:36.5. Beilenhoff U, Biering H, Blum R, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA): update 2018. Endoscopy. 2018; 50:1205–1234.6. Son BK, Kim BW, Kim WH, et al. Korean Society of Gastrointestinal Endoscopy guidelines for endoscope reprocessing. Clin Endosc. 2017; 50:143–147.7. Reprocessing Guideline Task Force, Petersen BT, Cohen J, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017; 85:282–294.e1.8. Barakat MT, Girotra M, Huang RJ, et al. Scoping the scope: endoscopic evaluation of endoscope working channels with a new high-resolution inspection endoscope (with video). Gastrointest Endosc. 2018; 88:601–611.e1.9. Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video). Gastrointest Endosc. 2019; 89:115–123.10. Otter JA, Vickery K, Walker JT, et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect. 2015; 89:16–27.11. Ren-Pei W, Hui-Jun X, Ke Q, et al. Correlation between the growth of bacterial biofilm in flexible endoscopes and endoscope reprocessing methods. Am J Infect Control. 2014; 42:1203–1206.12. Cheung DY, Jang BI, Kim SW, et al. Multidisciplinary and multisociety practice guideline on reprocessing flexible gastrointestinal endoscopes and endoscopic accessories. Clin Endosc. 2020; 53:276–285.13. Thaker AM, Kim S, Sedarat A, et al. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018; 88:612–619.14. Buss AJ, Been MH, Borgers RP, et al. Endoscope disinfection and its pitfalls--requirement for retrograde surveillance cultures. Endoscopy. 2008; 40:327–332.15. FDA/CDC/ASM Working Group on Duodenoscope Culturing. Duodenoscope surveillance sampling & culturing: reducing the risks of infection [Internet]. Atlanta (GA): Centers for Disease Control and Prevention;2018. [cited 2021 Mar 8]. Available from: https://www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html.16. ASGE Technology Committee, Komanduri S, Abu Dayyeh BK, et al. Technologies for monitoring the quality of endoscope reprocessing. Gastrointest Endosc. 2014; 80:369–373.17. Suzuki S, Nishimoto K, Igarashi T, et al. A novel bioluminescent cycling assay for ATP and AMP using pyruvate orthophosphate dikinase. In : Stanley PE, Kricka LJ, editors. Bioluminescence and chemiluminescence. Singapore: World Scientific;2002. p. 457–460.18. Ridtitid W, Pakvisal P, Chatsuwan T, et al. Performance characteristics and optimal cut-off value of triple adenylate nucleotides test versus adenosine triphosphate test as point-of-care testing for predicting inadequacy of duodenoscope reprocessing. J Hosp Infect. 2020; 106:348–356.19. Alrabaa SF, Nguyen P, Sanderson R, et al. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control. 2013; 41:562–564.20. Muscarella LF. Risk of transmission of carbapenem-resistant Enterobacteriaceae and related “superbugs” during gastrointestinal endoscopy. World J Gastrointest Endosc. 2014; 6:457–474.21. Fushimi R, Takashina M, Yoshikawa H, et al. Comparison of adenosine triphosphate, microbiological load, and residual protein as indicators for assessing the cleanliness of flexible gastrointestinal endoscopes. Am J Infect Control. 2013; 41:161–164.22. Alfa MJ, Fatima I, Olson N. Validation of adenosine triphosphate to audit manual cleaning of flexible endoscope channels. Am J Infect Control. 2013; 41:245–248.23. Alfa MJ, Olson N. Simulated-use validation of a sponge ATP method for determining the adequacy of manual cleaning of endoscope channels. BMC Res Notes. 2016; 9:258.24. Chu NS, McAlister D, Antonoplos PA. Natural bioburden levels detected on flexible gastrointestinal endoscopes after clinical use and manual cleaning. Gastrointest Endosc. 1998; 48:137–142.25. Ofstead CL, Hopkins KM, Eiland JE, et al. Widespread clinical use of simethicone, insoluble lubricants, and tissue glue during endoscopy: a call to action for infection preventionists. Am J Infect Control. 2019; 47:666–670.26. Liu TC, Peng CL, Wang HP, et al. SpyGlass application for duodenoscope working channel inspection: impact on the microbiological surveillance. World J Gastroenterol. 2020; 26:3767–3779.27. Bhatt S, Mehta P, Chen C, et al. Efficacy of low-temperature plasma-activated gas disinfection against biofilm on contaminated GI endoscope channels. Gastrointest Endosc. 2019; 89:105–114.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscope Reprocessing: Update on Controversial Issues
- Steps of Reprocessing and Equipments
- Does the Reprocessing of Endoscopes Have to Take Place Immediately after Pre-Cleaning? A First Evaluation
- Clinical Practice Guidelines for Endoscope Reprocessing
- Comparison on the Efficacy of Disinfectants Used in Automated Endoscope Reprocessors: PHMB-DBAC versus Orthophthalaldehyde