Clin Endosc.
2021 Jul;54(4):526-533. 10.5946/ce.2020.238.
Does the Reprocessing of Endoscopes Have to Take Place Immediately after Pre-Cleaning? A First Evaluation
- Affiliations
-
- 1Section for Hospital Hygiene and Environmental Health, Center for Infectious Diseases, Heidelberg University Hospital, Im Neuenheimer Feld 324, Heidelberg, Germany
- 2Infection Control Engineering, Center for Infectious Diseases, Heidelberg University Hospital, Im Neuenheimer Feld 324, Heidelberg, Germany
- 3Institute of Hygiene and Public Health, University Hospital Bonn, Venusberg-Campus 1, Bonn, Germany
- KMID: 2518855
- DOI: http://doi.org/10.5946/ce.2020.238
Abstract
- Background/Aims
The recommendations on the time interval between pre-cleaning and reprocessing of endoscopes differ in international guidelines, with a low level of evidence. The aim of this study was to investigate the influence of postponing reprocessing on the reprocessing quality after pre-cleaning the flexible endoscopes.
Methods
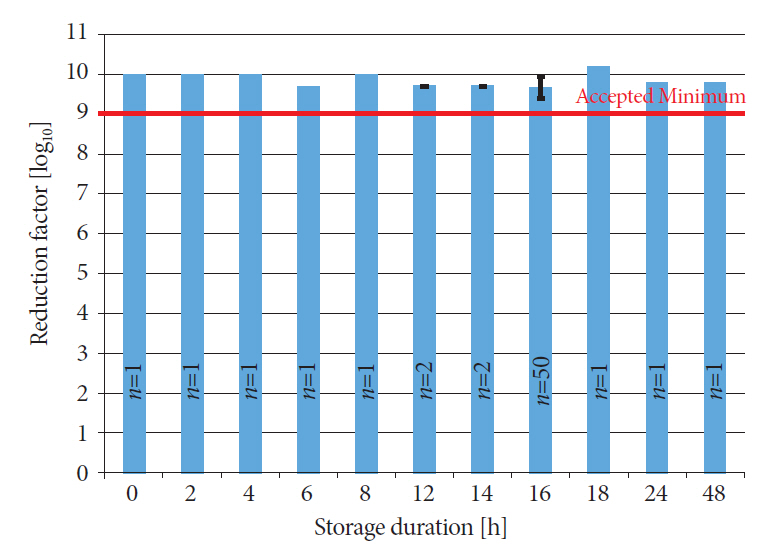
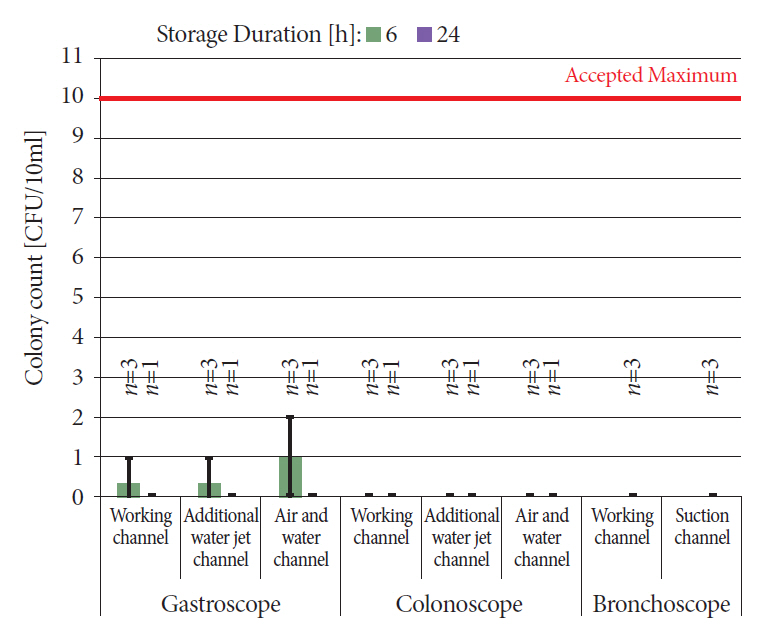
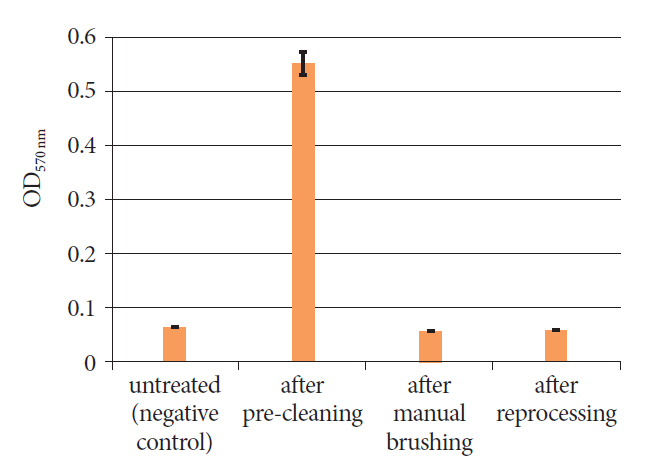
We reprocessed 124 standardized test tubes simulating endoscope channels after soiling and contamination and determined the reprocessing performance. In addition, we examined contaminated gastroscopes, colonoscopes, and bronchoscopes. The duration of interim storage after pre-cleaning was 16 h for 100 test tubes and up to 24 h for 18 endoscopes. We determined the residual protein content and germ load as markers for cleaning and disinfection performance. In addition, we determined biofilm formation by photometry of crystal violet staining.
Results
All test tubes and flexible endoscopes showed residual protein content and germ load significantly below legally prescribed threshold values, independent of the interval between pre-cleaning and reprocessing.
Conclusions
Our findings indicate that flexible endoscopes could be stored overnight after pre-cleaning without any influence on the quality of reprocessing. While ensuring patient safety, this could simplify logistical processes and enable cost savings.
Keyword
Figure
Cited by 1 articles
-
Endoscopes that Complete Pre-Cleaning may be Stored Overnight until Next Morning for the Subsequent Reprocessing
Soo-Jeong Cho
Clin Endosc. 2021;54(4):449-450. doi: 10.5946/ce.2021.135.
Reference
-
1. Kumarage J, Khonyongwa K, Khan A, Desai N, Hoffman P, Taori SK. Transmission of multi-drug resistant Pseudomonas aeruginosa between two flexible ureteroscopes and an outbreak of urinary tract infection: the fragility of endoscope decontamination. J Hosp Infect. 2019; 102:89–94.
Article2. Naas T, Cuzon G, Babics A, et al. Endoscopy-associated transmission of carbapenem-resistant Klebsiella pneumoniae producing KPC-2 beta-lactamase. J Antimicrob Chemother. 2010; 65:1305–1306.3. Shimono N, Takuma T, Tsuchimochi N, et al. An outbreak of Pseudomonas aeruginosa infections following thoracic surgeries occurring via the contamination of bronchoscopes and an automatic endoscope reprocessor. J Infect Chemother. 2008; 14:418–423.
Article4. McCafferty CE, Aghajani MJ, Abi-Hanna D, Gosbell IB, Jensen SO. An update on gastrointestinal endoscopy-associated infections and their contributing factors. Ann Clin Microbiol Antimicrob. 2018; 17:36.
Article5. Shellnutt C. Advances in endoscope reprocessing technology and its impact on pathogen transmission. Gastroenterol Nurs. 2016; 39:457–465.
Article6. Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten. Bundesgesundheitsbl. 2012; 55:1244–1310.7. Canada PHA of. Infection prevention and control guideline for flexible gastrointestinal endoscopy and flexible bronchoscopy [Internet]. Ottawa: Public Health Agency of Canada;c2011. [updated 2011 Feb 10; cited 2021 May 31]. Available from: https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/infection-prevention-control-guideline-flexible-gastrointestinal-endoscopy-flexible-bronchoscopy.8. Reprocessing Guideline Task Force, Petersen BT, Cohen J, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017; 85:282–294.e1.
Article9. Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Association for professionals in infection control. Am J Infect Control. 2000; 28:138–155.10. Guide Technique - Traitement des endoscopes souples thermosensibles à canaux [Internet]. Paris: Ministère des affaires sociales et de la santé en France;c2019. [updated 2019 Aug 6; cited 2021 May 31]. Available from: https://solidarites-sante.gouv.fr/IMG/pdf/dgos_traitement_endoscopes.pdf.11. Professional Standard Handbook Cleaning and Disinfection Flexible Endoscopes Version 4.1 [Internet]. Netherlands: SFERD;c2017. [updated 2017 Sep; cited 2021 May 31]. Available from: https://www.infectiepreventieopleidingen.nl/downloads/SFERDHandbook4_1.pdf.12. Schweizerische Richtlinie zur Aufbereitung flexibler Endoskope [Internet]. Bern: Schweizerische Gesellschaft für Gastroenterologie (SGG);c2010. [cited 2021 May 31]. Available from: https://sggssg.ch/fileadmin/_migrated/content_uploads/Arzt_17_Schweizerische_Hygienerichtlinie.pdf.13. Richtlinien und Empfehlungen :: SVEP-ASPE [Internet]. [cited 2021 May 31]. Available from: https://svep-aspe.ch/fileadmin/user_upload/pdf/CH_Richtlinie_ESGE_2020_07_10.pdf.14. Son BK, Kim B-W, Kim WH, et al. Korean society of gastrointestinal endoscopy guidelines for endoscope reprocessing. Clin Endosc. 2017; 50:143–147.
Article15. Health Technical Memorandum 01-06: Decontamination of flexible endoscopes [Internet]. London: Department of Health & Social Care UK;c2016. [cited 2021 May 31]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/530418/HTM0106_PartA.pdf; 2016.16. 2020 Guidance on Decontamination of Equipment for Gastrointestinal Endoscopy [Internet]. London: The British Society of Gastroenterology;c2020. [cited 2021 May 31]. Available from: https://www.bsg.org.uk/clinical-resource/guidance-on-decontamination-of-equipment-for-gastrointestinal-endoscopy/.17. Infection control in Endoscopy [Internet]. Wales: Gastroenterological Society of Australia and Gastroenterological Nurses College of Australia;c2011. [cited 2021 June 21]. Available from: https://www.genca.org/public/5/files/Endoscopy_infection_control%20(low).pdf.18. Beilenhoff U, Biering H, Blum R, et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - update 2018. Endoscopy. 2018; 50:1205–1234.
Article19. Beilenhoff U. Welches Zeitfenster ist für die Aufbereitung zu empfehlen? Dürfen Endoskope länger oder gar über Nacht liegen bleiben? Wie ist bei infektiösen Patienten zu verfahren? Endo-Praxis. 2018; 34:116–120.
Article20. Beilenhoff U, Biering H, Blum R, et al. Prevention of multidrug-resistant infections from contaminated duodenoscopes: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA). Endoscopy. 2017; 49:1098–1106.
Article21. ISO/TS 15883-5:2005 Washer-disinfectors - Part 5: Test soils and methods for demonstrating cleaning efficacy [Internet]. Geneva: ISO;c2005. [cited 2021 May 31]. Available from: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/11/41175.html.22. Wehrl M, Kircheis U. Methods for testing the cleaning performance of washing-disinfection equipment for flexible endoscopes. 2011; 36:402–406.23. Kircheis U, Wehrl M. Method for testing the cleaning and disinfecting efficacy of washer-disinfectors for flexible endoscopes. Zentralsterilisation - Central Service. 2012; 20:240–249.24. Guideline of DGKH, DGSV and AKI for the validation and routine monitoring of automated cleaning and thermal disinfection processes for medical devices [Internet]. Wiesbaden: Zentral STERILISATION;c2017. [cited 2021 June 21]. Available from: https://shop.mhp-verlag.de/media/pdf/6b/d0/17/MHP_ZS-Supplement-ENG-2017_E-Paper59e49ae0660bc.pdf.25. Kampf G, Fliss PM, Martiny H. Is peracetic acid suitable for the cleaning step of reprocessing flexible endoscopes? World J Gastrointest Endosc. 2014; 6:390–406.
Article26. Günther F, Scherrer M, Kaiser SJ, DeRosa A, Mutters NT. Comparative testing of disinfectant efficacy on planktonic bacteria and bacterial biofilms using a new assay based on kinetic analysis of metabolic activity. J Appl Microbiol. 2017; 122:625–633.
Article27. O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998; 28:449–461.28. Stepanović S, Vuković D, Hola V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007; 115:891–899.
Article29. Alfa MJ, Degagne P, Olson N. Worst-case soiling levels for patient-used flexible endoscopes before and after cleaning. Am J Infect Control. 1999; 27:392–401.
Article30. Alfa MJ. Medical instrument reprocessing: current issues with cleaning and cleaning monitoring. Am J Infect Control. 2019; 47S:A10–A16.
Article31. Cattoir L, Vanzieleghem T, Florin L, et al. Surveillance of endoscopes: comparison of different sampling techniques. Infect Control Hosp Epidemiol. 2017; 38:1062–1069.
Article32. Shin SP, Kim WH. Recent update on microbiological monitoring of gastrointestinal endoscopes after high-level disinfection. Clin Endosc. 2015; 48:369–373.
Article33. Alfa MJ, Olson N. Physical and composition characteristics of clinical secretions compared with test soils used for validation of flexible endoscope cleaning. J Hosp Infect. 2016; 93:83–88.
Article34. Chen X, Ling P, Duan R, Zhang T. Effects of heparosan and heparin on the adhesion and biofilm formation of several bacteria in vitro. Carbohydrate Polymers. 2012; 88:1288–1292.
Article35. ISO 15883-5 Washer-disinfectors - Part 5: Performance requirements and test method criteria for demonstrating cleaning efficacy [Internet]. Geneva: ISO;c2021. [cited 2021 May 31]. Available from: https://www.iso.org/standard/68297.html.