Blood Res.
2022 Sep;57(3):244-246. 10.5045/br.2022.2022128.
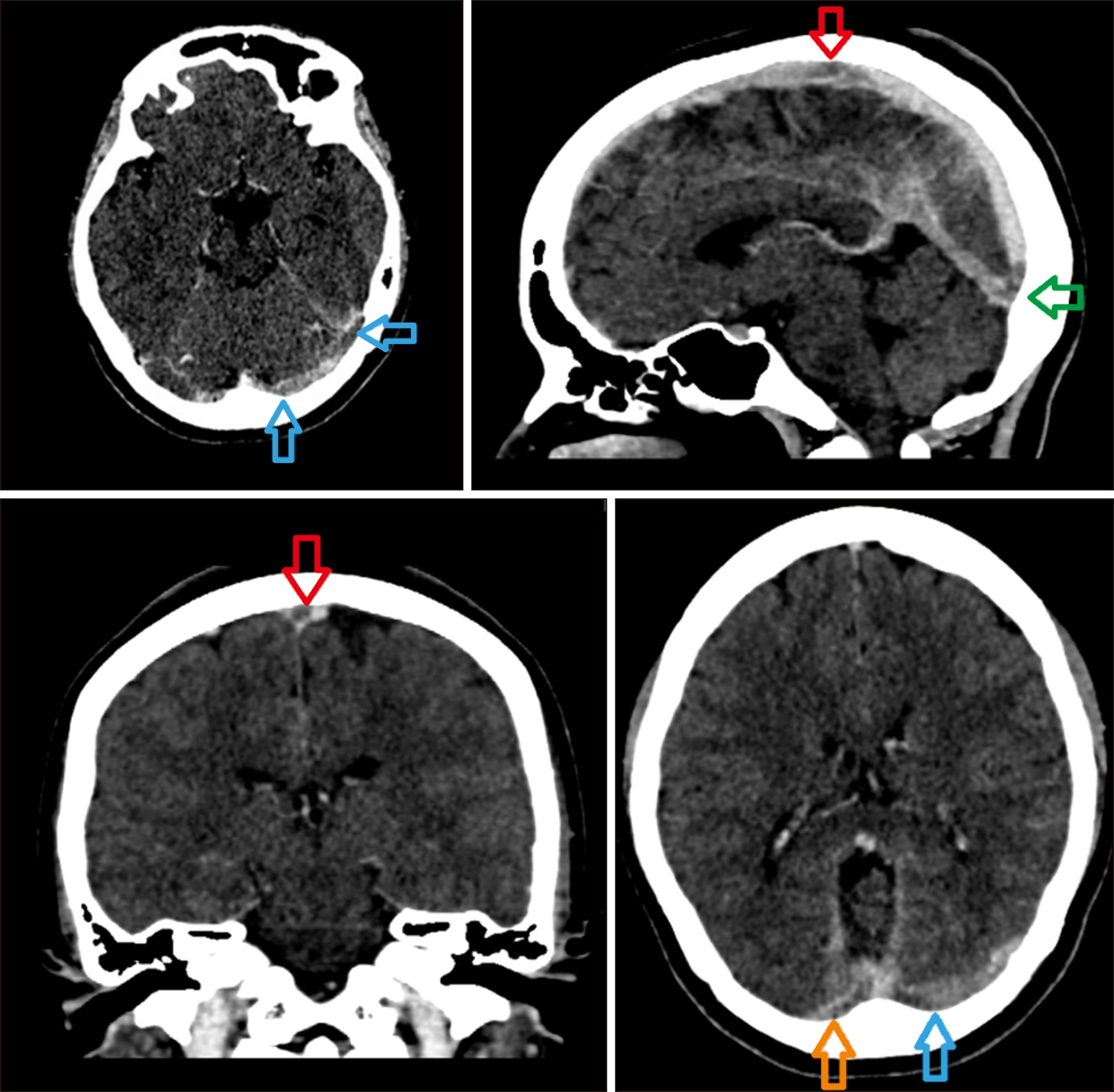
Vaccine-induced immune thrombotic thrombocytopenia (VITT) with cerebral venous sinus thrombosis (CVST): a case report from Malaysia
- Affiliations
-
- 1Departments of Internal Medicine, Hospital Sultanah Aminah, Johor Bahru, Johor, Malaysia
- 2Departments of Radiology, Hospital Sultanah Aminah, Johor Bahru, Johor, Malaysia
- KMID: 2533255
- DOI: http://doi.org/10.5045/br.2022.2022128
Figure
Reference
-
1. Greinacher A, Langer F, Makris M, et al. 2022; Vaccine-induced immune thrombotic thrombocytopenia (VITT): update on diagnosis and management considering different resources. J Thromb Haemost. 20:149–56. DOI: 10.1111/jth.15572. PMID: 34693641. PMCID: PMC8646430.
Article2. Schultz NH, Sørvoll IH, Michelsen AE, et al. 2021; Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 384:2124–30. DOI: 10.1056/NEJMoa2104882. PMID: 33835768. PMCID: PMC8112568.
Article3. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. 2021; Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 384:2092–101. DOI: 10.1056/NEJMoa2104840. PMID: 33835769. PMCID: PMC8095372.4. Scully M, Singh D, Lown R, et al. 2021; Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 384:2202–11. DOI: 10.1056/NEJMoa2105385. PMID: 33861525. PMCID: PMC8112532.5. Pavord S, Scully M, Hunt BJ, et al. 2021; Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 385:1680–9. DOI: 10.1056/NEJMoa2109908. PMID: 34379914.
Article6. Boonyawat K, Angchaisuksiri P. 2022; Vaccine-induced immune thrombotic thrombocytopenia with ChAdOx1 nCoV-19 is rare in Asia. Res Pract Thromb Haemost. 6:e12644. DOI: 10.1002/rth2.12644. PMID: 35071968. PMCID: PMC8760608. PMID: b10dc7fe07da4a2eb6f4b3b0e8fdfcd5.
Article7. Lee LH, Gallus A, Jindal R, Wang C, Wu CC. 2017; Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 117:2243–60. DOI: 10.1160/TH17-02-0134. PMID: 29212112.8. Boonyawat K, Angchaisuksiri P, Aryurachai K, Chaiyaroj S, Ahmadi Z, Chong BH. 2014; Low prevalence of heparin-induced thrombocytopenia after cardiac surgery in Thai patients. Thromb Res. 134:957–62. DOI: 10.1016/j.thromres.2014.08.020. PMID: 25204999.
Article9. National Pharmaceutical Regulatory Agency, Ministry of Health, Malaysia. 2022. Summary report on adverse events following immunisation of COVID-19 vaccines in Malaysia. National Pharmaceutical Regulatory Agency (NPRA);Petaling Jaya, Malaysia: at https://www.npra.gov.my/index.php/en/. Accessed April 20, 2022.10. Rizk JG, Gupta A, Sardar P, et al. 2021; Clinical characteristics and pharmacological management of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: a review. JAMA Cardiol. 6:1451–60. DOI: 10.1001/jamacardio.2021.3444. PMID: 34374713.
Article11. Klok FA, Pai M, Huisman MV, Makris M. 2022; Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 9:e73–80. DOI: 10.1016/S2352-3026(21)00306-9. PMID: 34774202. PMCID: PMC8585488.12. Arepally GM, Ortel TL. 2021; Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 138:293–8. DOI: 10.1182/blood.2021012152. PMID: 34323940. PMCID: PMC8172307.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review
- Thrombosis and severe acute respiratory syndrome coronavirus 2 vaccines: vaccine-induced immune thrombotic thrombocytopenia
- A Case Report of Thrombotic Thrombocytopenia After ChAdOx1 nCov-19 Vaccination and Heparin Use During Hemodialysis
- Cerebral Venous Sinus Thrombosis in an Adolescent Presenting with Headache
- Cerebral Venous Sinus Thrombosis with Meningitis and Septicemia due to Haemophilus influenzae Type f in an Immunocompetent Child