J Korean Med Sci.
2022 Mar;37(10):e75. 10.3346/jkms.2022.37.e75.
A Case Report of Thrombotic Thrombocytopenia After ChAdOx1 nCov-19 Vaccination and Heparin Use During Hemodialysis
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2526981
- DOI: http://doi.org/10.3346/jkms.2022.37.e75
Abstract
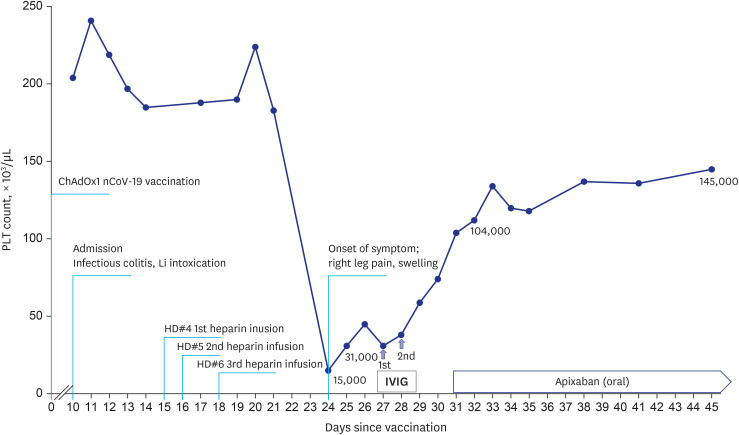
- Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare but life-threatening complication. VITT strongly mimics heparin-induced thrombocytopenia (HIT) and shares clinical features. Heparin is commonly used to prevent coagulation during hemodialysis. Therefore, nephrologists might encounter patients needing dialysis with a history of heparin exposure who developed thrombotic thrombocytopenia after vaccination. A 70-year-old male presented with acute kidney injury and altered mental status due to lithium intoxication. He needed consecutive hemodialysis using heparin. Deep vein thrombosis of left lower extremity and accompanying severe thrombocytopenia of 15,000/µL on 24 days after vaccination and at the same time, nine days after heparin use. Anti-platelet factor 4 antibody test was positive. Anticoagulation with apixaban and intravenous immunoglobulin (IVIG) infusion resolved swelling of his left calf and thrombocytopenia. There were no definitive diagnostic tools capable of differentiating between VITT and HIT in this patient. Although VITT and HIT share treatment with IVIG and non-heparin anticoagulation, distinguishing between VITT and HIT will make it possible to establish a follow-up vaccination plan in a person who has had a thrombocytopenic thrombotic event. Further research is needed to develop the tools to make a clear distinction between the clinical syndromes.
Keyword
Figure
Reference
-
1. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021; 325(24):2448–2456. PMID: 33929487.
Article2. Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021; 385(18):1680–1689. PMID: 34379914.
Article3. Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus pandemic (COVID-19). Updated 2021. Accessed October 11, 2021. https://ourworldindata.org/coronavirus .4. Omer SB, Yildirim I, Forman HP. Herd immunity and implications for SARS-CoV-2 control. JAMA. 2020; 324(20):2095–2096. PMID: 33074293.
Article5. Gupta A, Sardar P, Cash ME, Milani RV, Lavie CJ. COVID-19 vaccine- induced thrombosis and thrombocytopenia-a commentary on an important and practical clinical dilemma. Prog Cardiovasc Dis. 2021; 67:105–107. PMID: 34019911.
Article6. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021; 384(22):2092–2101. PMID: 33835769.
Article7. Bourguignon A, Arnold DM, Warkentin TE, Smith JW, Pannu T, Shrum JM, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021; 385(8):720–728. PMID: 34107198.
Article8. UK Medicines and Healthcare Products Regulatory Agency (MHRA). Coronavirus vaccine - weekly summary of Yellow Card reporting. Updated 2021. Accessed November 17, 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting .9. World Health Organization (WHO). Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). Updated 2021. Accessed November 17, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-TTS-2021.1 .10. McGonagle D, De Marco G, Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun. 2021; 121:102662. PMID: 34051613.
Article11. Arepally GM, Padmanabhan A. Heparin-induced thrombocytopenia: a focus on thrombosis. Arterioscler Thromb Vasc Biol. 2021; 41(1):141–152. PMID: 33267665.12. Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007; 83(983):575–582. PMID: 17823223.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Livedo reticularis following administration of ChAdOx1 nCoV-19 vaccine (AZD1222): a report of two cases
- A Case Report of Immune Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination
- Deep vein thrombosis and acute hepatitis after ChAdOx1 nCov-19 vaccination in a Charcot-MarieTooth patient: a case report
- Two Case Reports of Leukocytoclastic Vasculitis after ChAdOx1 nCoV-19 Vaccine
- Acute Myocardial Infarction with Microthrombi in Cardiac Small Vessels after COVID-19 Vaccination (ChAdOx1 nCov-19): A Case Report