Ann Rehabil Med.
2022 Aug;46(4):172-184. 10.5535/arm.22053.
Quantitative Analysis in Cervical Spinal Cord Injury Patients Using Diffusion Tensor Imaging and Tractography
- Affiliations
-
- 1Department of Rehabilitation Medicine, Dankook University Hospital, Cheonan, Korea
- 2Department of Rehabilitation Medicine, Dankook University College of Medicine, Cheonan, Korea
- 3Department of Nanobiomedical Science & BK21 NBM Research Center for Regenerative Medicine, Dankook University, Cheonan, Korea
- KMID: 2532950
- DOI: http://doi.org/10.5535/arm.22053
Abstract
Objective
To investigate the clinical usefulness of diffusion tensor imaging (DTI) and tractography in the prediction of outcomes after traumatic cervical spinal cord injury (SCI) and to assess whether the predictability is different between DTI and tractography administered before and after surgery.
Methods
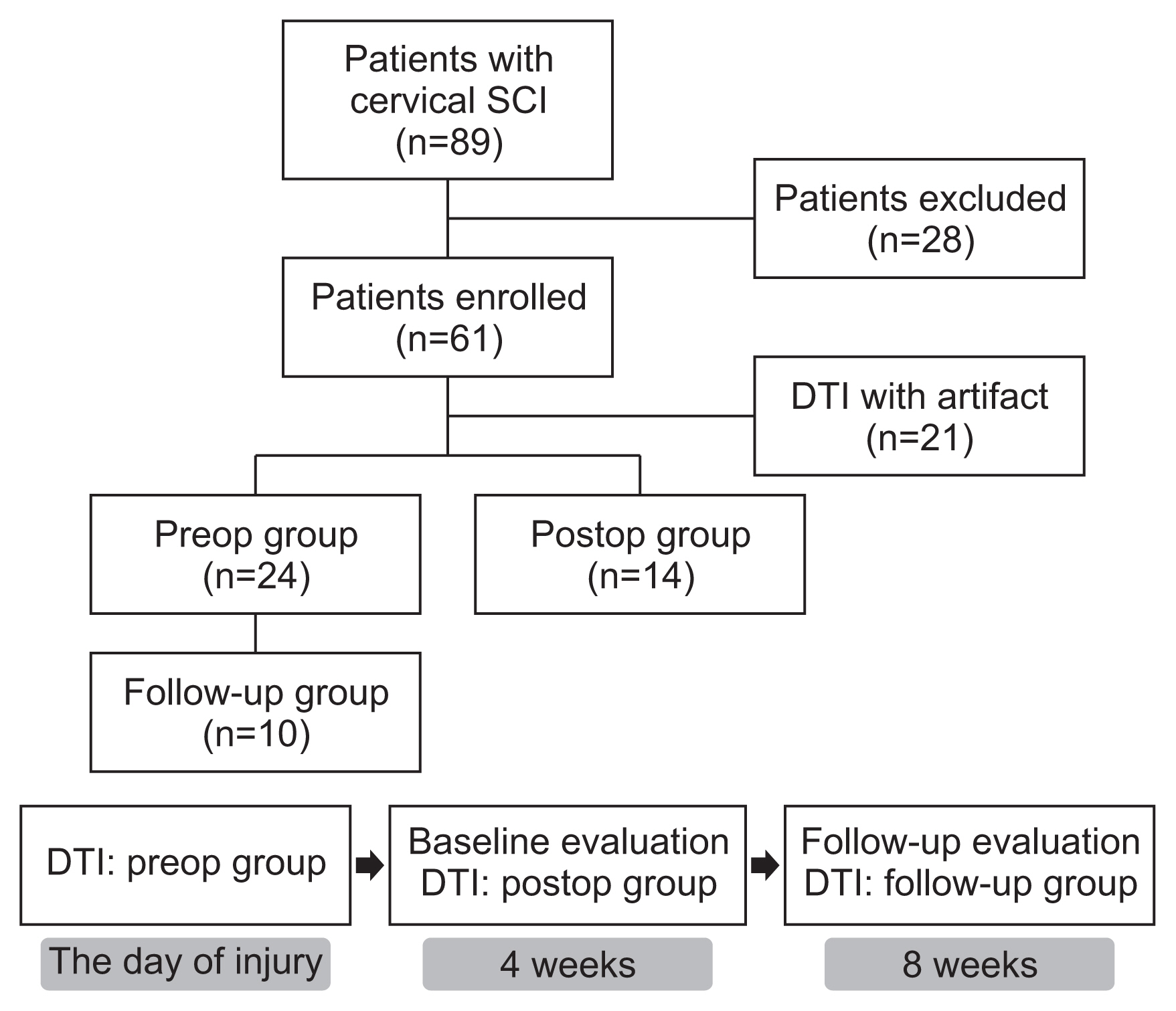
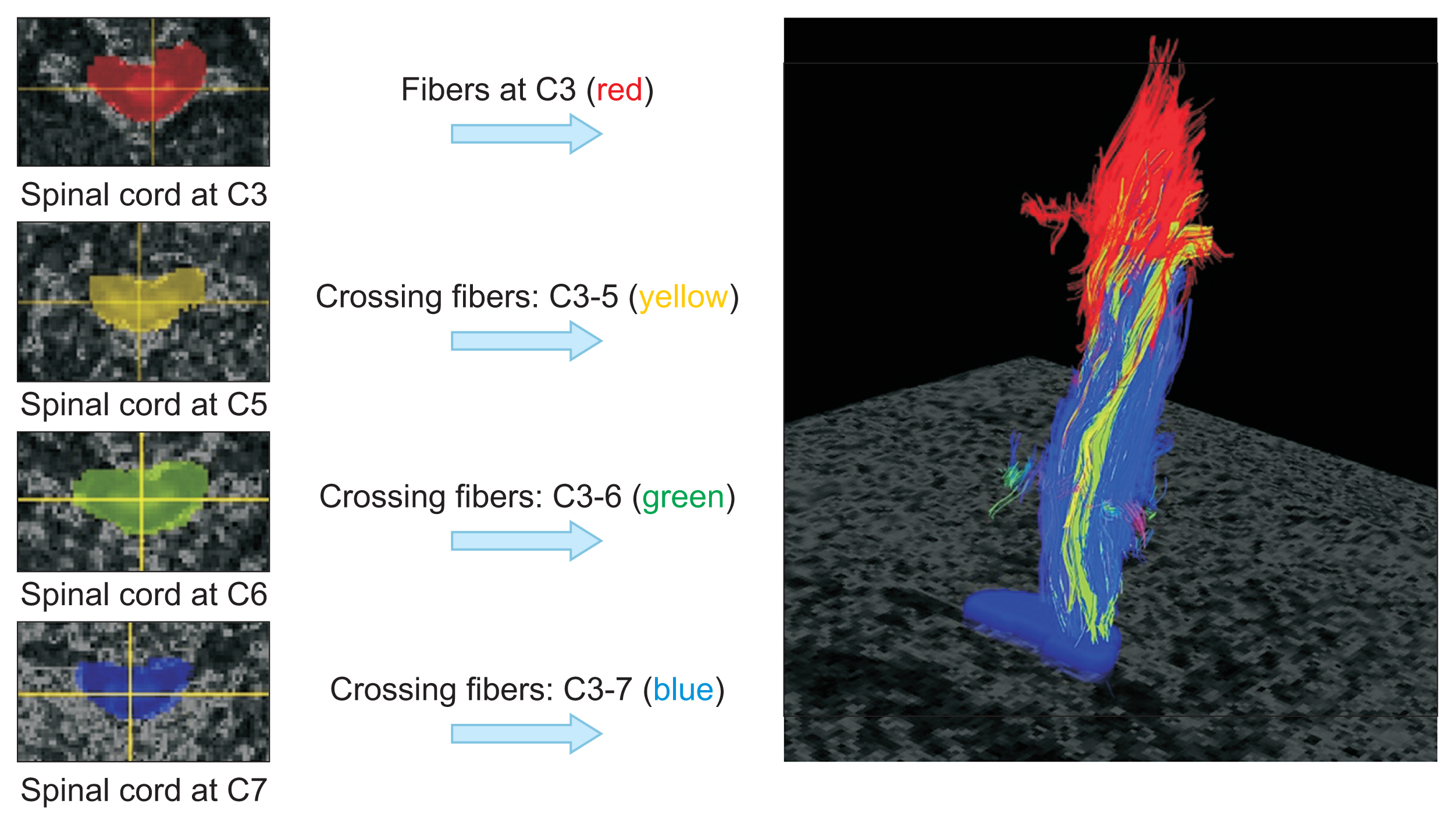
Sixty-one subjects with traumatic cervical SCI were randomly assigned to preop or postop groups and received DTI accordingly. Among the patients who had DTI before surgery, we assigned 10 patients who had received repeated DTI examinations at 8 weeks after injury to the follow-up group. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) values were obtained from DTI, and imaginary fiber and crossing fiber numbers were calculated from the tractography. Neurological status and functional status were assessed at 4 and 8 weeks after SCI.
Results
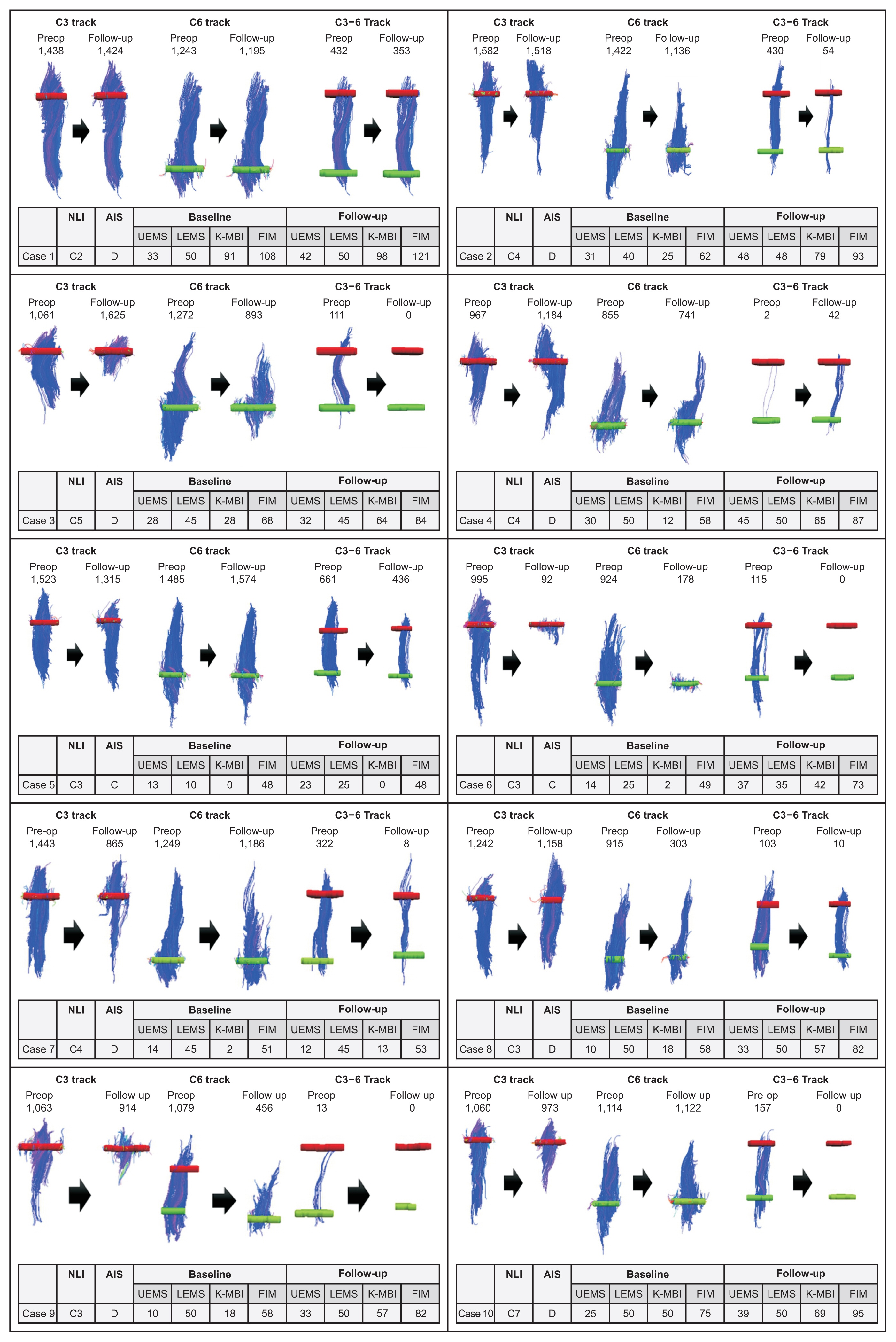
The neurologic and functional statuses of both groups improved after 4 weeks. Out of the initial 61 patients who were enrolled in the study, the failure rate of DTI image analysis was significantly higher in the postop group (n=17, 41.5%) than in the preop group (n=6, 20%). The FA values and fiber numbers in the preop group tended to be higher than those in the postop group, whereas ADC values were lower in the preop group. When comparing the tractographic findings in the follow-up group, imaginary fiber numbers at the C6 and C7 levels and crossing fiber numbers from the C3 to C6 levels were significantly decreased after surgery. Several DTI and tractographic parameters (especially the ADC value at the C4 level and imaginary fiber numbers at the C6 level) showed significant correlations with neurologic and functional statuses in both the preop and postop groups. These findings were most prominent when DTI and physical examination were simultaneously performed.
Conclusion
Preoperative DTI and tractography demonstrated better FA and ADC values with lower interpretation failure rates than those obtained after surgery, whereas postoperative data significantly reflected the patient’s clinical state at the time of evaluation. Therefore, DTI and tractography could be useful in predicting clinical outcomes after traumatic cervical SCI and should be interpreted separately before and after spine surgery.
Figure
Reference
-
1. Kirshblum SC, Priebe MM, Ho CH, Scelza WM, Chiodo AE, Wuermser LA. Spinal cord injury medicine. 3. Rehabilitation phase after acute spinal cord injury. Arch Phys Med Rehabil. 2007; 88(3 Suppl 1):S62–70.
Article2. Sato T, Kokubun S, Rijal KP, Ojima T, Moriai N, Hashimoto M, et al. Prognosis of cervical spinal cord injury in correlation with magnetic resonance imaging. Paraplegia. 1994; 32:81–5.
Article3. Aarabi B, Sansur CA, Ibrahimi DM, Simard JM, Hersh DS, Le E, et al. Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of ASIA Impairment Scale grade conversion following decompressive surgery in cervical spinal cord injury. Neurosurgery. 2017; 80:610–20.
Article4. Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008; 7:615–25.
Article5. Wheeler-Kingshott CA, Hickman SJ, Parker GJ, Ciccarelli O, Symms MR, Miller DH, et al. Investigating cervical spinal cord structure using axial diffusion tensor imaging. Neuroimage. 2002; 16:93–102.
Article6. Toktas ZO, Tanrikulu B, Koban O, Kilic T, Konya D. Diffusion tensor imaging of cervical spinal cord: a quantitative diagnostic tool in cervical spondylotic myelopathy. J Craniovertebr Junction Spine. 2016; 7:26–30.
Article7. Chang Y, Jung TD, Yoo DS, Hyun JK. Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma. 2010; 27:2033–40.
Article8. Rutman AM, Peterson DJ, Cohen WA, Mossa-Basha M. Diffusion tensor imaging of the spinal cord: clinical value, investigational applications, and technical limitations. Curr Probl Diagn Radiol. 2018; 47:257–69.
Article9. Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011; 34:535–46.
Article10. Kwon S, Hartzema AG, Duncan PW, Min-Lai S. Disability measures in stroke: relationship among the Barthel Index, the Functional Independence Measure, and the Modified Rankin Scale. Stroke. 2004; 35:918–23.11. Jones DK, Leemans A. Diffusion tensor imaging. Methods Mol Biol. 2011; 711:127–44.
Article12. Rajasekaran S, Kanna RM, Shetty AP. Diffusion tensor imaging of the spinal cord and its clinical applications. J Bone Joint Surg Br. 2012; 94:1024–31.
Article13. Koskinen EA, Hakulinen U, Brander AE, Luoto TM, Ylinen A, Ohman JE. Clinical correlates of cerebral diffusion tensor imaging findings in chronic traumatic spinal cord injury. Spinal Cord. 2014; 52:202–8.
Article14. Kaushal M, Shabani S, Budde M, Kurpad S. Diffusion tensor imaging in acute spinal cord injury: a review of animal and human studies. J Neurotrauma. 2019; 36:2279–86.
Article15. Martin Noguerol T, Barousse R, Amrhein TJ, Royuela-Del-Val J, Montesinos P, Luna A. Optimizing diffusion-tensor imaging acquisition for spinal cord assessment: physical basis and technical adjustments. Radiographics. 2020; 40:403–27.16. Loy DN, Kim JH, Xie M, Schmidt RE, Trinkaus K, Song SK. Diffusion tensor imaging predicts hyperacute spinal cord injury severity. J Neurotrauma. 2007; 24:979–90.
Article17. Kim JH, Loy DN, Liang HF, Trinkaus K, Schmidt RE, Song SK. Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn Reson Med. 2007; 58:253–60.
Article18. Kim JH, Loy DN, Wang Q, Budde MD, Schmidt RE, Trinkaus K, et al. Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J Neurotrauma. 2010; 27:587–98.
Article19. Li XH, Li JB, He XJ, Wang F, Huang SL, Bai ZL. Timing of diffusion tensor imaging in the acute spinal cord injury of rats. Sci Rep. 2015; 5:12639.
Article20. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems: 2018 annual report - complete public version [Internet]. Birmingham, AL: National Spinal Cord Injury Statistical Center;2018. [cited 2022 Jul 7]. Available from: https://www.nscisc.uab.edu/public/2018 Annual Report - Complete Public Version.pdf .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Metrics as a Potential Prognostic Biomarker in Cervical Myelopathy
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications
- Reversible Psychosis Caused by Disconnection of the Limbic System: Clinical Reasoning Using Diffusion Tensor Tractography
- Diffusion Tensor Imaging and Cerebrospinal Fluid Flow Study of Cine Phase Contrast in Normal Cervical Spinal Cords
- Diffusion Tensor Imaging in Inflammatory and Neoplastic Intramedullary Spinal Cord Lesions: Focusing on Fiber Tracking