Korean J Physiol Pharmacol.
2022 Sep;26(5):377-387. 10.4196/kjpp.2022.26.5.377.
Anti-cancer effects of fenbendazole on 5-fluorouracil-resistant colorectal cancer cells
- Affiliations
-
- 1Department of Histology, College of Medicine, Jeju National University, Jeju 63243, Korea
- 2Department of Cellular and Molecular Medicine, Chosun University School of Medicine, Gwangju 61452, Korea
- 3Department of Anatomy, College of Medicine, Jeju National University, Jeju 63243, Korea
- KMID: 2532767
- DOI: http://doi.org/10.4196/kjpp.2022.26.5.377
Abstract
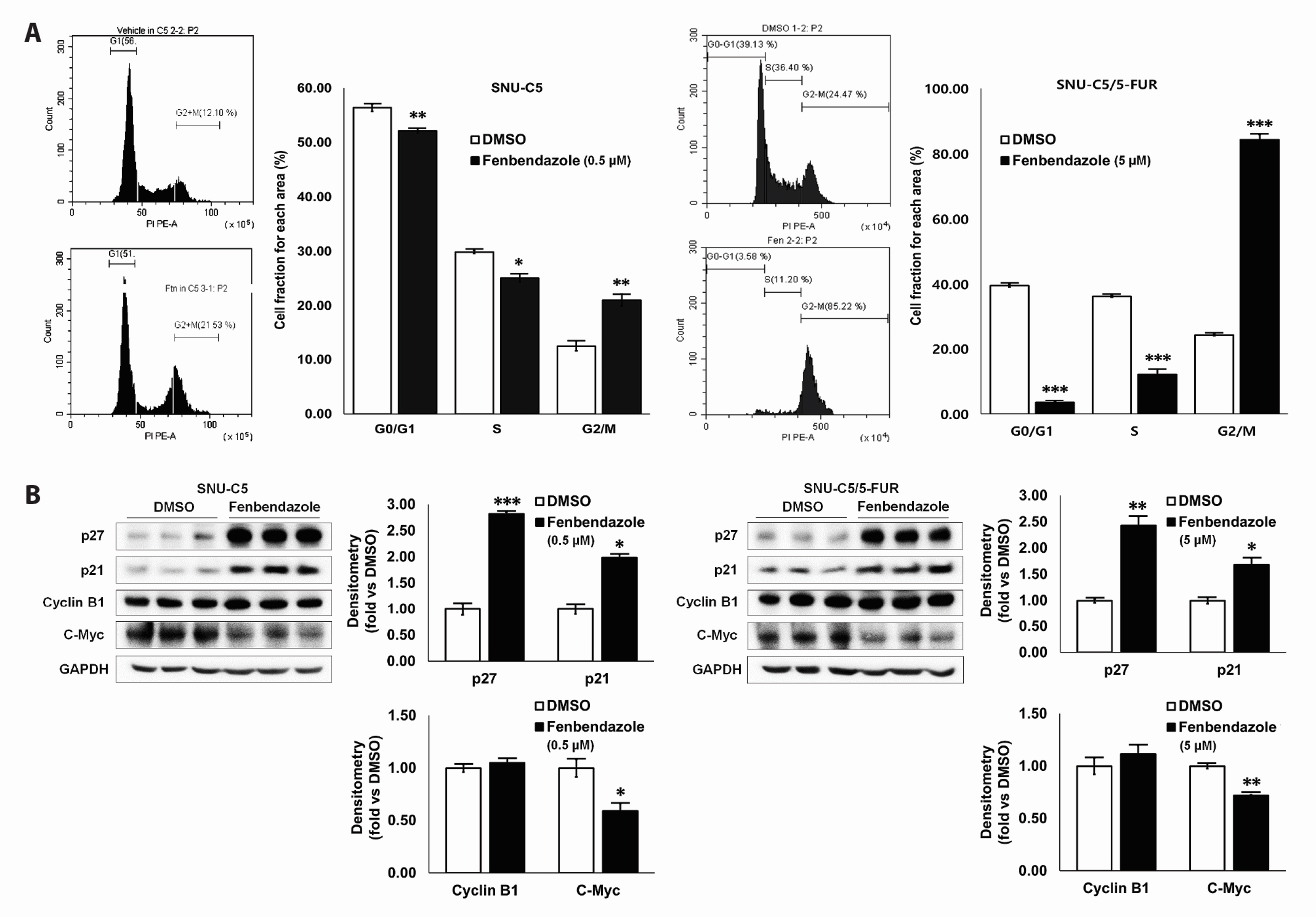
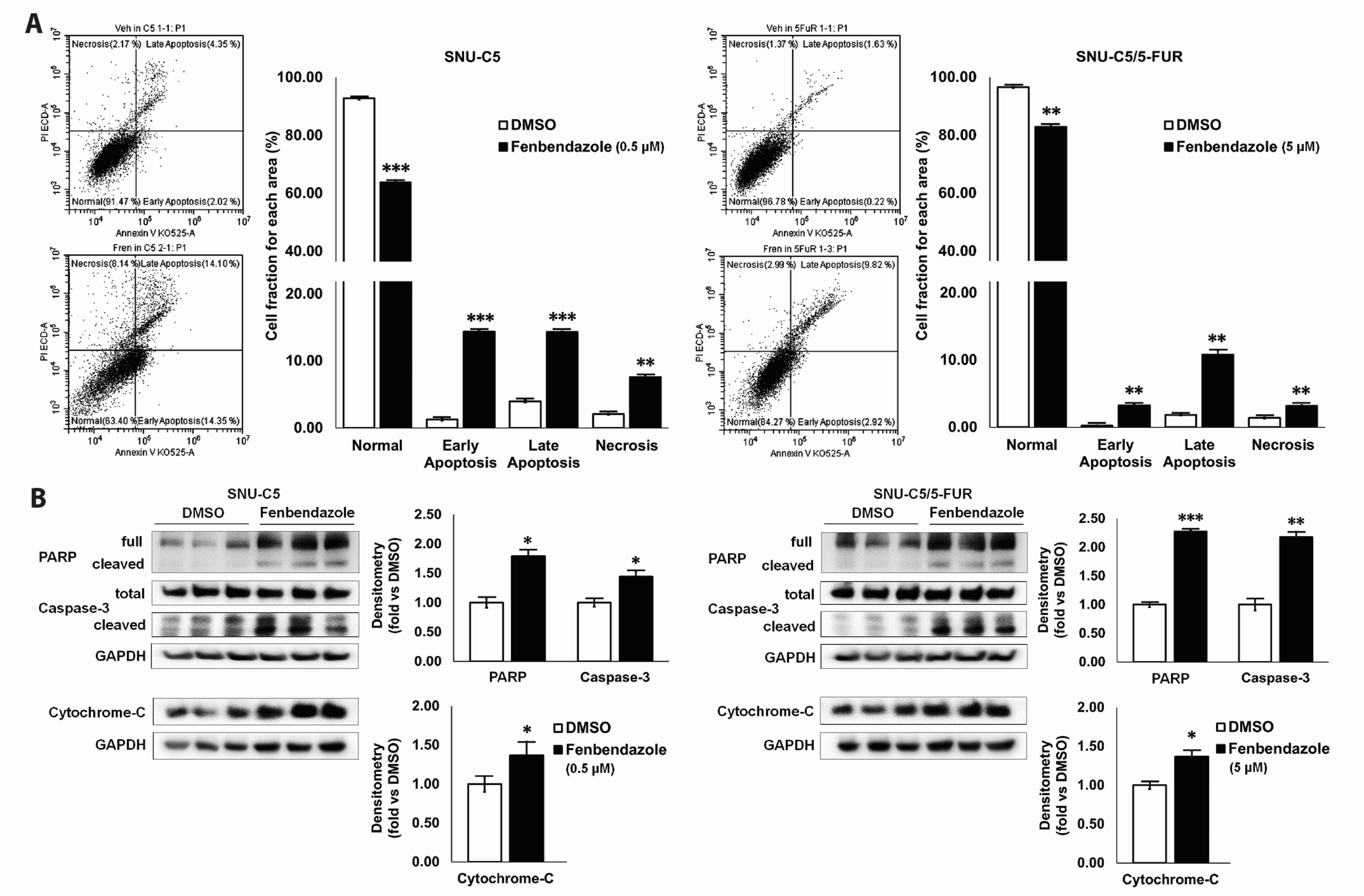
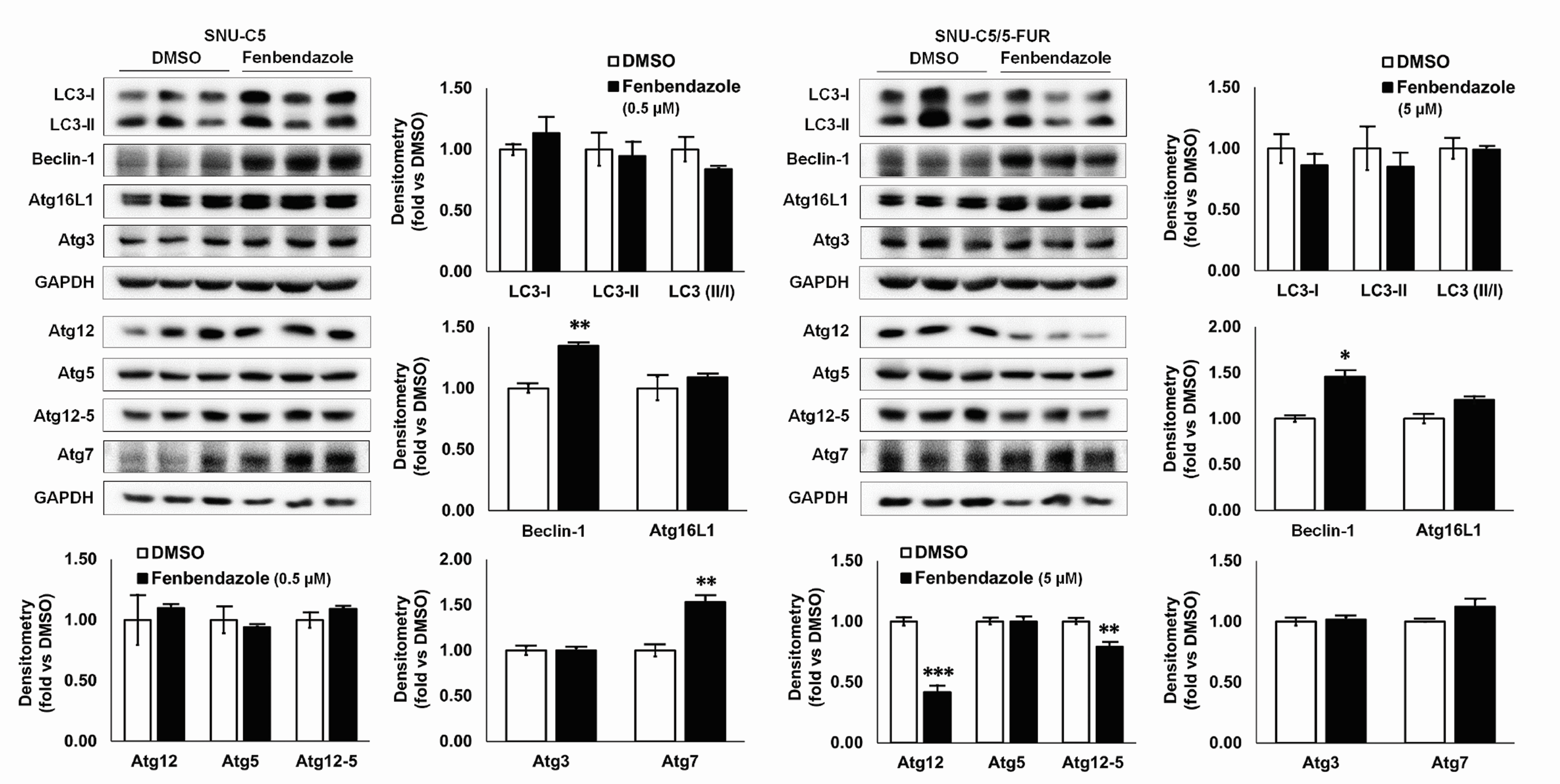
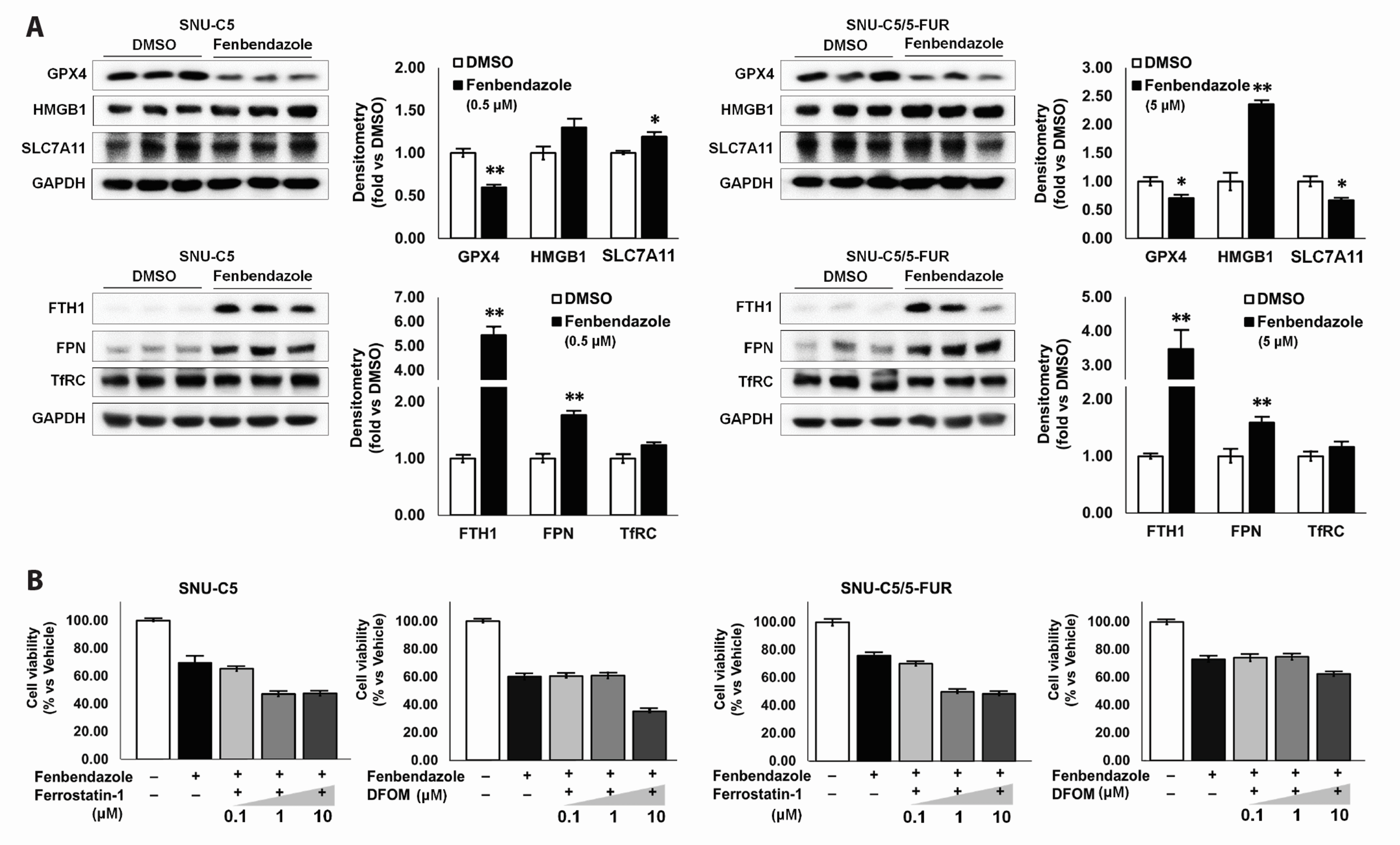
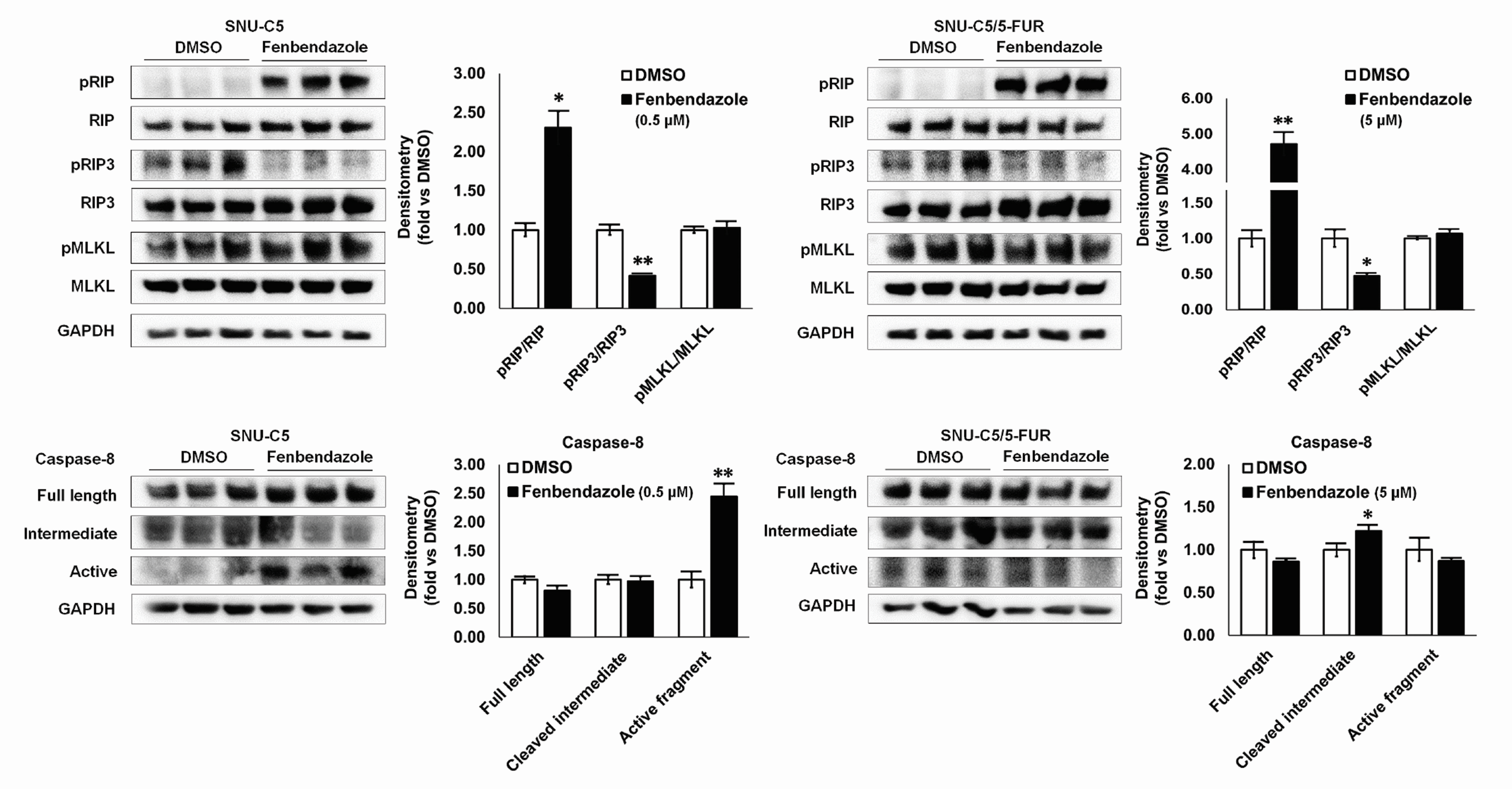
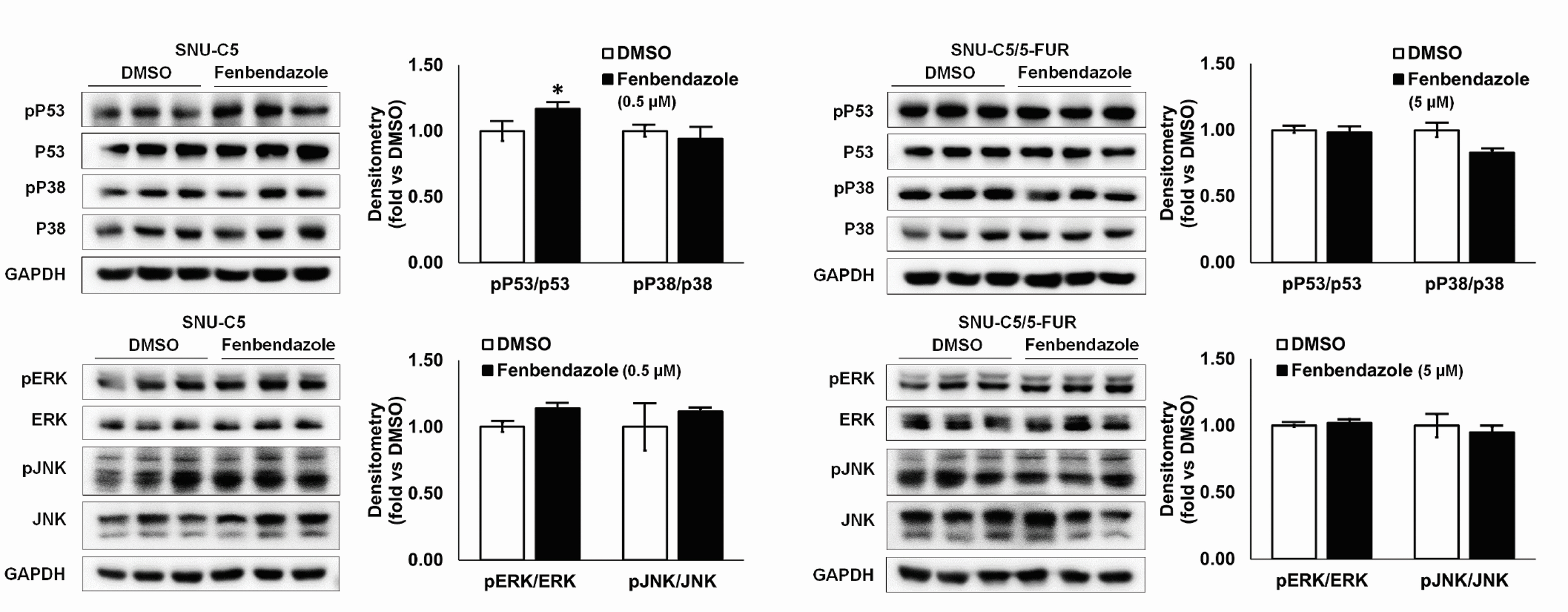
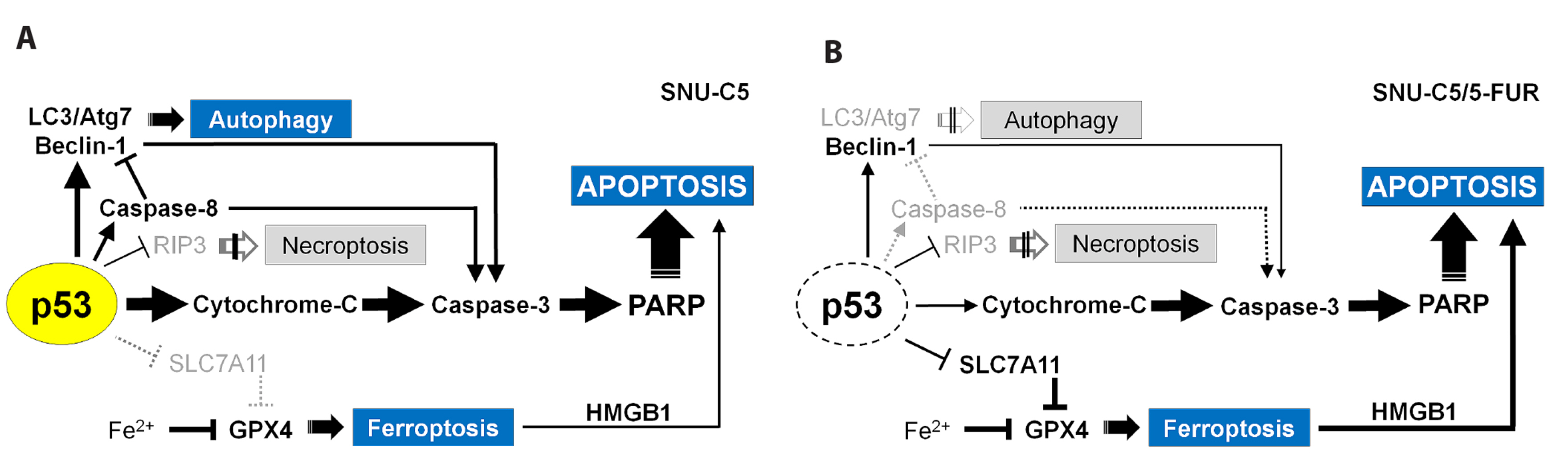
- Benzimidazole anthelmintic agents have been recently repurposed to overcome cancers resistant to conventional therapies. To evaluate the anti-cancer effects of benzimidazole on resistant cells, various cell death pathways were investigated in 5-fluorouracil-resistant colorectal cancer cells. The viability of wild-type and 5-fluorouracil-resistant SNU-C5 colorectal cancer cells was assayed, followed by Western blotting. Flow cytometry assays for cell death and cell cycle was also performed to analyze the anti-cancer effects of benzimidazole. When compared with albendazole, fenbendazole showed higher susceptibility to 5-fluorouracil-resistant SNU-C5 cells and was used in subsequent experiments. Flow cytometry revealed that fenbendazole significantly induces apoptosis as well as cell cycle arrest at G2/ M phase on both cells. When compared with wild-type SNU-C5 cells, 5-fluorouracilresistant SNU-C5 cells showed reduced autophagy, increased ferroptosis and ferroptosis-augmented apoptosis, and less activation of caspase-8 and p53. These results suggest that fenbendazole may be a potential alternative treatment in 5-fluorouracilresistant cancer cells, and the anticancer activity of fenbendazole does not require p53 in 5-fluorouracil-resistant SNU-C5 cells.
Figure
Reference
-
1. Son DS, Lee ES, Adunyah SE. 2020; The antitumor potentials of benzimidazole anthelmintics as repurposing drugs. Immune Netw. 20:e29. DOI: 10.4110/in.2020.20.e29. PMID: 32895616. PMCID: PMC7458798.
Article2. Mukhopadhyay T, Sasaki J, Ramesh R, Roth JA. 2002; Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin Cancer Res. 8:2963–2969. PMID: 12231542.3. Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth JA, Mukhopadhyay T. 2002; The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol Cancer Ther. 1:1201–1209. PMID: 12479701.4. Martarelli D, Pompei P, Baldi C, Mazzoni G. 2008; Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother Pharmacol. 61:809–817. DOI: 10.1007/s00280-007-0538-0. PMID: 17581752.
Article5. Dogra N, Mukhopadhyay T. 2012; Impairment of the ubiquitin-proteasome pathway by methyl N-(6-phenylsulfanyl-1H-benzimidazol-2-yl)carbamate leads to a potent cytotoxic effect in tumor cells: a novel antiproliferative agent with a potential therapeutic implication. J Biol Chem. 287:30625–30640. DOI: 10.1074/jbc.M111.324228. PMID: 22745125. PMCID: PMC3436308.
Article6. Lin S, Yang L, Yao Y, Xu L, Xiang Y, Zhao H, Wang L, Zuo Z, Huang X, Zhao C. 2019; Flubendazole demonstrates valid antitumor effects by inhibiting STAT3 and activating autophagy. J Exp Clin Cancer Res. 38:293. DOI: 10.1186/s13046-019-1303-z. PMID: 31287013. PMCID: PMC6615228. PMID: 3e2c66b97c604ee1b9fbb07e1c470dc9.
Article7. Michaelis M, Agha B, Rothweiler F, Löschmann N, Voges Y, Mittelbronn M, Starzetz T, Harter PN, Abhari BA, Fulda S, Westermann F, Riecken K, Spek S, Langer K, Wiese M, Dirks WG, Zehner R, Cinatl J, Wass MN, Cinatl J Jr. 2015; Identification of flubendazole as potential anti-neuroblastoma compound in a large cell line screen. Sci Rep. 5:8202. DOI: 10.1038/srep08202. PMID: 25644037. PMCID: PMC4314641.
Article8. Heo DS. 2020; Anthelmintics as potential anti-cancer drugs? J Korean Med Sci. 35:e75. DOI: 10.3346/jkms.2020.35.e75. PMID: 32056406. PMCID: PMC7025903.
Article9. Yamaguchi T, Shimizu J, Oya Y, Horio Y, Hida T. 2021; Drug-induced liver injury in a patient with nonsmall cell lung cancer after the self-administration of fenbendazole based on social media information. Case Rep Oncol. 14:886–891. DOI: 10.1159/000516276. PMID: 34248555. PMCID: PMC8255718.
Article10. Chiang RS, Syed AB, Wright JL, Montgomery B, inlavs S Sr. 2021; Fenbendazole enhancing anti-tumor effect: a case series. Clin Oncol Case Rep. 4:2. https://www.scitechnol.com/peer-review/fenbendazole-enhancing-antitumor-effect-a-case-series-P3SV.pdf.11. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. Erratum in: CA Cancer J Clin. 2020;70:313. DOI: 10.3322/caac.21492. PMID: 30207593.
Article12. Azwar S, Seow HF, Abdullah M, Faisal Jabar M, Mohtarrudin N. 2021; Recent updates on mechanisms of resistance to 5-fluorouracil and reversal strategies in colon cancer treatment. Biology (Basel). 10:854. DOI: 10.3390/biology10090854. PMID: 34571731. PMCID: PMC8466833. PMID: 63c56935834c487a856258521353921b.
Article13. Pan MH, Lai CS, Wu JC, Ho CT. 2011; Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res. 55:32–45. DOI: 10.1002/mnfr.201000412. PMID: 21207511.
Article14. Fridman JS, Lowe SW. 2003; Control of apoptosis by p53. Oncogene. 22:9030–9040. DOI: 10.1038/sj.onc.1207116. PMID: 14663481.
Article15. Li XL, Zhou J, Chen ZR, Chng WJ. 2015; P53 mutations in colorectal cancer- molecular pathogenesis and pharmacological reactivation. World J Gastroenterol. 21:84–93. DOI: 10.3748/wjg.v21.i1.84. PMID: 25574081. PMCID: PMC4284363.
Article16. Thornton TM, Rincon M. 2009; Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 5:44–51. DOI: 10.7150/ijbs.5.44. PMID: 19159010. PMCID: PMC2610339.
Article17. Grossi V, Peserico A, Tezil T, Simone C. 2014; p38α MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol. 20:9744–9758. DOI: 10.3748/wjg.v20.i29.9744. PMID: 25110412. PMCID: PMC4123363.
Article18. Kim EJ, Kang JI, Kwak JW, et al. 2015; The anticancer effect of (1S,2S,3E,7E,11E)-3,7,11, 15-cembratetraen-17,2-olide(LS-1) through the activation of TGF-β signaling in SNU-C5/5-FU, fluorouracil-resistant human colon cancer cells. Mar Drugs. 13:1340–1359. DOI: 10.3390/md13031340. PMID: 25786063. PMCID: PMC4377987.
Article19. Moon D, Kang HK, Kim J, Yoon SP. 2020; Yeast extract induces apoptosis and cell cycle arrest via activating p38 signal pathway in colorectal cancer cells. Ann Clin Lab Sci. 50:31–44. PMID: 32161010.20. Arkun Y. 2016; Dynamic modeling and analysis of the cross-talk between insulin/AKT and MAPK/ERK signaling pathways. PLoS One. 11:e0149684. DOI: 10.1371/journal.pone.0149684. PMID: 26930065. PMCID: PMC4773096.
Article21. Nygren P, Fryknäs M, Agerup B, Larsson R. 2013; Repositioning of the anthelmintic drug mebendazole for the treatment for colon cancer. J Cancer Res Clin Oncol. 139:2133–2140. DOI: 10.1007/s00432-013-1539-5. PMID: 24135855. PMCID: PMC3825534.
Article22. Al-Douh MH, Sahib HB, Osman H, Abd Hamid S, Salhimi SM. 2012; Anti-proliferation effects of benzimidazole derivatives on HCT-116 colon cancer and MCF-7 breast cancer cell lines. Asian Pac J Cancer Prev. 13:4075–4079. DOI: 10.7314/APJCP.2012.13.8.4075. PMID: 23098519.
Article23. Chen K, Chu BZ, Liu F, Li B, Gao CM, Li LL, Sun QS, Shen ZF, Jiang YY. 2015; New benzimidazole acridine derivative induces human colon cancer cell apoptosis in vitro via the ROS-JNK signaling pathway. Acta Pharmacol Sin. 36:1074–1084. DOI: 10.1038/aps.2015.44. PMID: 26235743. PMCID: PMC4561968.
Article24. Králová V, Hanušová V, Rudolf E, Čáňová K, Skálová L. 2016; Flubendazole induces mitotic catastrophe and senescence in colon cancer cells in vitro. J Pharm Pharmacol. 68:208–218. DOI: 10.1111/jphp.12503. PMID: 26730435.
Article25. Hanušová V, Skálová L, Králová V, Matoušková P. 2018; The effect of flubendazole on adhesion and migration in SW480 and SW620 colon cancer cells. Anticancer Agents Med Chem. 18:837–846. DOI: 10.2174/1871520618666171213141911. PMID: 29237383.
Article26. Williamson T, Bai RY, Staedtke V, Huso D, Riggins GJ. 2016; Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget. 7:68571–68584. DOI: 10.18632/oncotarget.11851. PMID: 27612418. PMCID: PMC5356574.
Article27. Duan Q, Liu Y, Rockwell S. 2013; Fenbendazole as a potential anticancer drug. Anticancer Res. 33:355–362. PMID: 23393324. PMCID: PMC3580766.28. Doudican N, Rodriguez A, Osman I, Orlow SJ. 2008; Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res. 6:1308–1315. DOI: 10.1158/1541-7786.MCR-07-2159. PMID: 18667591.
Article29. Bai RY, Staedtke V, Aprhys CM, Gallia GL, Riggins GJ. 2011; Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro Oncol. 13:974–982. DOI: 10.1093/neuonc/nor077. PMID: 21764822. PMCID: PMC3158014.
Article30. Mrkvová Z, Uldrijan S, Pombinho A, Bartůněk P, Slaninová I. 2019; Benzimidazoles downregulate Mdm2 and MdmX and activate p53 in MdmX overexpressing tumor cells. Molecules. 24:2152. DOI: 10.3390/molecules24112152. PMID: 31181622. PMCID: PMC6600429.
Article31. Tang Y, Liang J, Wu A, Chen Y, Zhao P, Lin T, Zhang M, Xu Q, Wang J, Huang Y. 2017; Co-delivery of trichosanthin and albendazole by nano-self-assembly for overcoming tumor multidrug-resistance and metastasis. ACS Appl Mater Interfaces. 9:26648–26664. Erratum in: ACS Appl Mater Interfaces. 2020;12:3275. DOI: 10.1021/acsami.7b05292. PMID: 28741923.
Article32. Kim JW, Kim SH, Mariappan R, Moon D, Kim J, Yoon SP. 2021; Anti-cancer effects of the aqueous extract of Orostachys japonica A. Berger on 5-fluorouracil-resistant colorectal cancer via MAPK signalling pathways in vitro and in vivo. J Ethnopharmacol. 280:114412. DOI: 10.1016/j.jep.2021.114412. PMID: 34265383.
Article33. Feng R, Li S, Lu C, Andreas C, Stolz DB, Mapara MY, Lentzsch S. 2011; Targeting the microtubular network as a new antimyeloma strategy. Mol Cancer Ther. 10:1886–1896. DOI: 10.1158/1535-7163.MCT-11-0234. PMID: 21825007.
Article34. Wales CT, Taylor FR, Higa AT, McAllister HA, Jacobs AT. 2015; ERK-dependent phosphorylation of HSF1 mediates chemotherapeutic resistance to benzimidazole carbamates in colorectal cancer cells. Anticancer Drugs. 26:657–666. DOI: 10.1097/CAD.0000000000000231. PMID: 25811962.
Article35. Dogra N, Kumar A, Mukhopadhyay T. 2018; Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci Rep. 8:11926. DOI: 10.1038/s41598-018-30158-6. PMID: 30093705. PMCID: PMC6085345.
Article36. Yang WS, iRamaratnam R Sr, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. 2014; Regulation of ferroptotic cancer cell death by GPX4. Cell. 156:317–331. DOI: 10.1016/j.cell.2013.12.010. PMID: 24439385. PMCID: PMC4076414.
Article37. Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, et al. 2014; Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 16:1180–1191. DOI: 10.1038/ncb3064. PMID: 25402683. PMCID: PMC4894846.
Article38. Latunde-Dada GO. 2017; Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 1861:1893–1900. DOI: 10.1016/j.bbagen.2017.05.019. PMID: 28552631.
Article39. Lee YS, Lee DH, Jeong SY, Park SH, Oh SC, Park YS, Yu J, Choudry HA, Bartlett DL, Lee YJ. 2019; Ferroptosis-inducing agents enhance TRAIL-induced apoptosis through upregulation of death receptor 5. J Cell Biochem. 120:928–939. DOI: 10.1002/jcb.27456. PMID: 30160785. PMCID: PMC6249082.40. Kaczmarek A, Vandenabeele P, Krysko DV. 2013; Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 38:209–223. DOI: 10.1016/j.immuni.2013.02.003. PMID: 23438821.
Article41. Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. 2014; Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 16:55–65. Erratum in: Nat Cell Biol. 2014;16:200. DOI: 10.1038/ncb2883. PMID: 24316671. PMCID: PMC8369836.
Article42. Nirmala JG, Lopus M. 2020; Cell death mechanisms in eukaryotes. Cell Biol Toxicol. 36:145–164. DOI: 10.1007/s10565-019-09496-2. PMID: 31820165.
Article43. Zhong B, Liu M, Bai C, Ruan Y, Wang Y, Qiu L, Hong Y, Wang X, Li L, Li B. 2020; Caspase-8 induces lysosome-associated cell death in cancer cells. Mol Ther. 28:1078–1091. DOI: 10.1016/j.ymthe.2020.01.022. PMID: 32053770. PMCID: PMC7132614.
Article44. Younis NS, Ghanim AMH, Saber S. 2019; Mebendazole augments sensitivity to sorafenib by targeting MAPK and BCL-2 signalling in n-nitrosodiethylamine-induced murine hepatocellular carcinoma. Sci Rep. 9:19095. DOI: 10.1038/s41598-019-55666-x. PMID: 31836811. PMCID: PMC6911098.
Article45. Zhang F, Li Y, Zhang H, Huang E, Gao L, Luo W, Wei Q, Fan J, Song D, Liao J, Zou Y, Liu F, Liu J, Huang J, Guo D, Ma C, Hu X, Li L, Qu X, Chen L, et al. 2017; Anthelmintic mebendazole enhances cisplatin's effect on suppressing cell proliferation and promotes differentiation of head and neck squamous cell carcinoma (HNSCC). Oncotarget. 8:12968–12982. DOI: 10.18632/oncotarget.14673. PMID: 28099902. PMCID: PMC5355070.
Article46. Xu D, Tian W, Jiang C, Huang Z, Zheng S. 2019; The anthelmintic agent oxfendazole inhibits cell growth in non-small cell lung cancer by suppressing c-Src activation. Mol Med Rep. 19:2921–2926. DOI: 10.3892/mmr.2019.9897.
Article47. Mirzayans R, Andrais B, Scott A, Murray D. 2012; New insights into p53 signaling and cancer cell response to DNA damage: implications for cancer therapy. J Biomed Biotechnol. 2012:170325. DOI: 10.1155/2012/170325. PMID: 22911014. PMCID: PMC3403320.
Article48. Wang DB, Kinoshita C, Kinoshita Y, Morrison RS. 2014; p53 and mitochondrial function in neurons. Biochim Biophys Acta. 1842:1186–1197. DOI: 10.1016/j.bbadis.2013.12.015. PMID: 24412988. PMCID: PMC4074561.
Article49. Moulder DE, Hatoum D, Tay E, Lin Y, McGowan EM. 2018; The roles of p53 in mitochondrial dynamics and cancer metabolism: the pendulum between survival and death in breast cancer? Cancers (Basel). 10:189. DOI: 10.3390/cancers10060189. PMID: 29890631. PMCID: PMC6024909.
Article50. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. 2019; The molecular machinery of regulated cell death. Cell Res. 29:347–364. DOI: 10.1038/s41422-019-0164-5. PMID: 30948788. PMCID: PMC6796845.
Article51. Kang R, Kroemer G, Tang D. 2019; The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 133:162–168. DOI: 10.1016/j.freeradbiomed.2018.05.074. PMID: 29800655. PMCID: PMC6251771.
Article52. Lei G, Mao C, Yan Y, Zhuang L, Gan B. 2021; Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell. 12:836–857. DOI: 10.1007/s13238-021-00841-y. PMID: 33891303. PMCID: PMC8563889.
Article53. Ren LW, Li W, Zheng XJ, Liu JY, Yang YH, Li S, Zhang S, Fu WQ, Xiao B, Wang JH, Du GH. 2022; Benzimidazoles induce concurrent apoptosis and pyroptosis of human glioblastoma cells via arresting cell cycle. Acta Pharmacol Sin. 43:194–208. DOI: 10.1038/s41401-021-00752-y. PMID: 34433903.
Article54. Elayapillai S, Ramraj S, Benbrook DM, Bieniasz M, Wang L, Pathuri G, Isingizwe ZR, Kennedy AL, Zhao YD, Lightfoot S, Hunsucker LA, Gunderson CC. 2021; Potential and mechanism of mebendazole for treatment and maintenance of ovarian cancer. Gynecol Oncol. 160:302–311. DOI: 10.1016/j.ygyno.2020.10.010. PMID: 33131904. PMCID: PMC8820236.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of reactive oxygen species in the anti-cancer activity of fenbendazole, a benzimidazole anthelmintic
- Phase II study of 5-fluorouracil and recombinant interferon-gamma in patients with advanced colorectal cancer
- Cellular Prion Protein Enhances Drug Resistance of Colorectal Cancer Cells via Regulation of a Survival Signal Pathway
- The Endoplasmic Reticulum Stress Response Mediates Shikonin-Induced Apoptosis of 5-Fluorouracil–Resistant Colorectal Cancer Cells
- Sensitization of 5-Fluorouracil-Resistant SNUC5 Colon Cancer Cells to Apoptosis by α-Mangostin