Korean J Physiol Pharmacol.
2022 Sep;26(5):335-345. 10.4196/kjpp.2022.26.5.335.
Tanshinone IIA reduces pyroptosis in rats with coronary microembolization by inhibiting the TLR4/MyD88/NF-κB/NLRP3 pathway
- Affiliations
-
- 1Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University & Guangxi Key Laboratory Base of Precision Medicine in CardioCerebrovascular Diseases Control and Prevention & Guangxi Clinical Research Center for Cardio-Cerebrovascular Diseases, Nanning 530021, People’s Republic of China
- KMID: 2532763
- DOI: http://doi.org/10.4196/kjpp.2022.26.5.335
Abstract
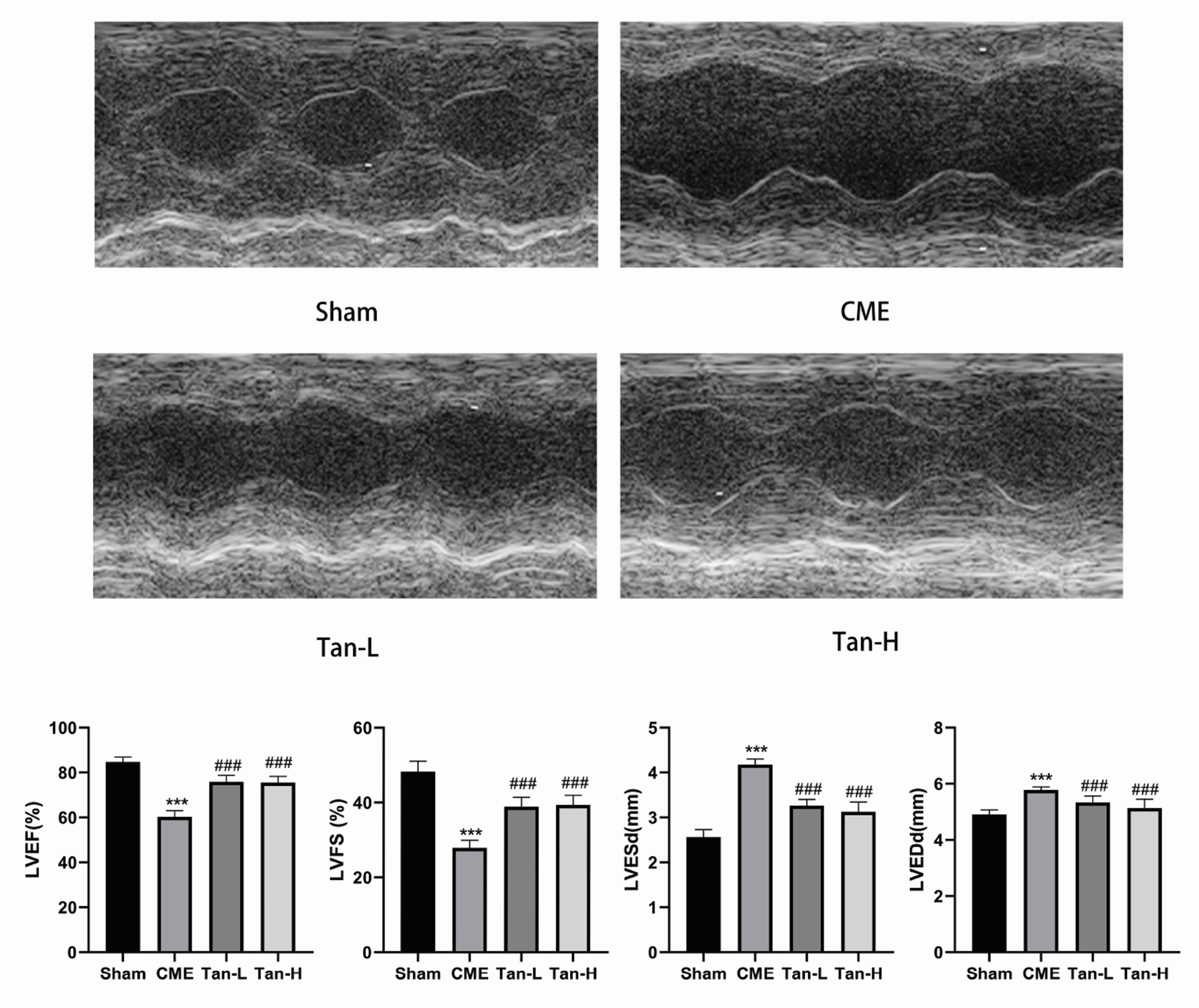
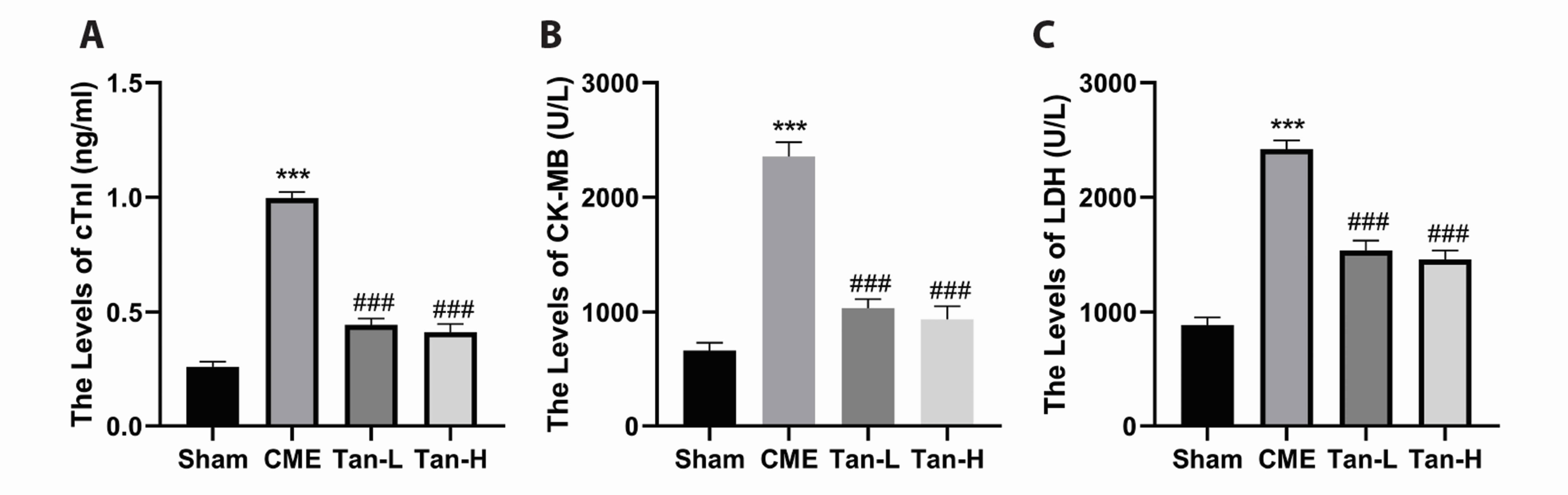
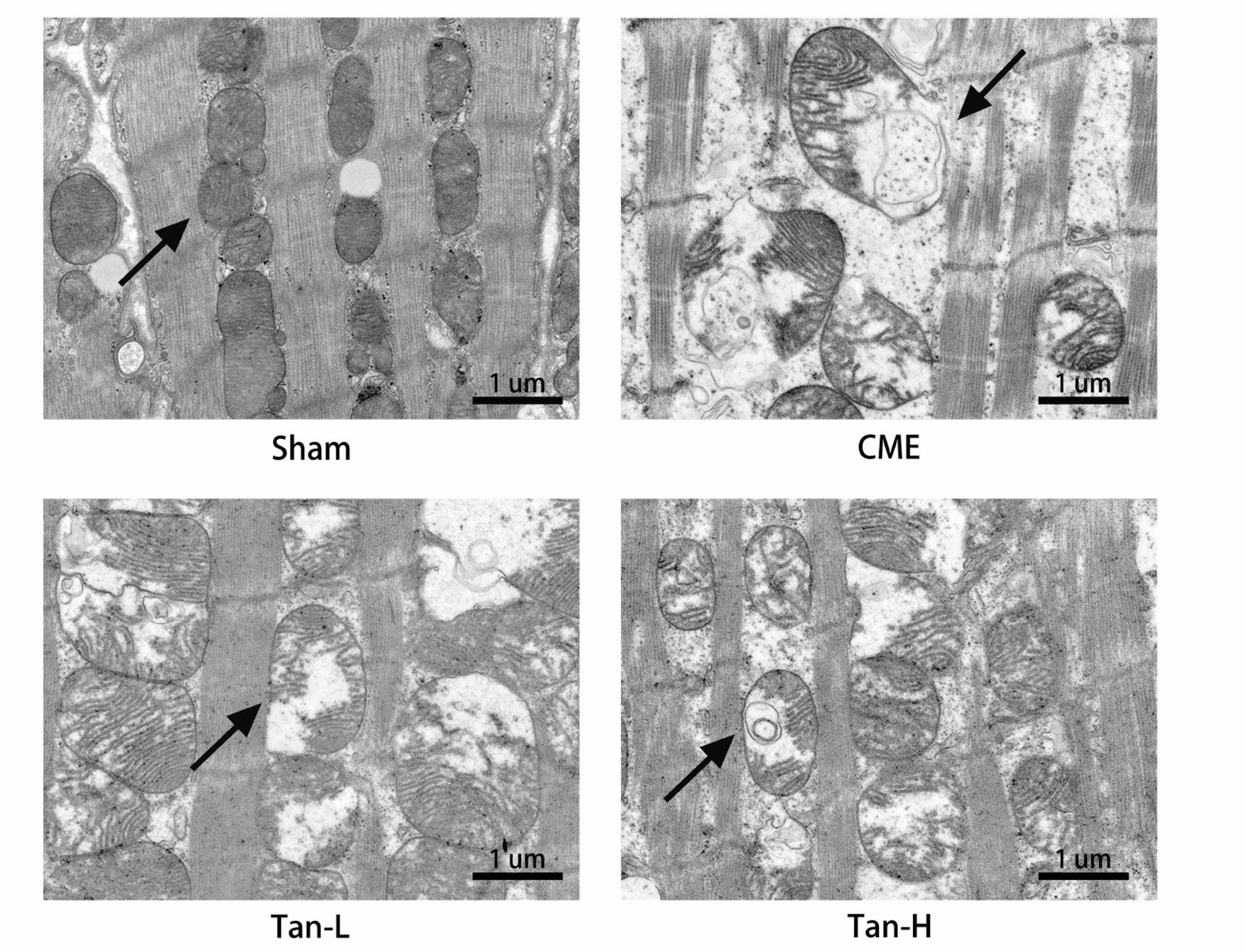
- Pyroptosis is an inflammatory form of programmed cell death that is linked with invading intracellular pathogens. Cardiac pyroptosis has a significant role in coronary microembolization (CME), thus causing myocardial injury. Tanshinone IIA (Tan IIA) has powerful cardioprotective effects. Hence, this study aimed to identify the effect of Tan IIA on CME and its underlying mechanism. Forty Sprague–Dawley (SD) rats were randomly grouped into sham, CME, CME + low-dose Tan IIA, and CME + high-dose Tan IIA groups. Except for the sham group, polyethylene microspheres (42 µm) were injected to establish the CME model. The Tan-L and Tan-H groups received intraperitoneal Tan IIA for 7 days before CME. After CME, cardiac function, myocardial histopathology, and serum myocardial injury markers were assessed. The expression of pyroptosis-associated molecules and TLR4/MyD88/NF-κB/NLRP3 cascade was evaluated by qRT-PCR, Western blotting, ELISA, and IHC. Relative to the sham group, CME group's cardiac functions were significantly reduced, with a high level of serum myocardial injury markers, and microinfarct area. Also, the levels of caspase-1 p20, GSDMD-N, IL-18, IL-1β, TLR4, MyD88, p-NF-κB p65, NLRP3, and ASC expression were increased. Relative to the CME group, the Tan-H and Tan-L groups had considerably improved cardiac functions, with a considerably low level of serum myocardial injury markers and microinfarct area. Tan IIA can reduce the levels of pyroptosis-associated mRNA and protein, which may be caused by inhibiting TLR4/MyD88/NF-κB/NLRP3 cascade. In conclusion, Tanshinone IIA can suppress cardiomyocyte pyroptosis probably through modulating the TLR4/MyD88/NF-κB/NLRP3 cascade, lowering cardiac dysfunction, and myocardial damage.
Figure
Reference
-
1. Bahrmann P, Werner GS, Heusch G, Ferrari M, Poerner TC, Voss A, Figulla HR. 2007; Detection of coronary microembolization by Doppler ultrasound in patients with stable angina pectoris undergoing elective percutaneous coronary interventions. Circulation. 115:600–608. DOI: 10.1161/CIRCULATIONAHA.106.660779. PMID: 17261655.
Article2. Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R. 2009; Coronary microembolization: from bedside to bench and back to bedside. Circulation. 120:1822–1836. DOI: 10.1161/CIRCULATIONAHA.109.888784. PMID: 19884481.3. Otto S, Seeber M, Fujita B, Kretzschmar D, Ferrari M, Goebel B, Figulla HR, Poerner TC. 2012; Microembolization and myonecrosis during elective percutaneous coronary interventions in diabetic patients: an intracoronary Doppler ultrasound study with 2-year clinical follow-up. Basic Res Cardiol. 107:289. DOI: 10.1007/s00395-012-0289-x. PMID: 22850870.
Article4. Heusch G, Skyschally A, Kleinbongard P. 2018; Coronary microembolization and microvascular dysfunction. Int J Cardiol. 258:17–23. DOI: 10.1016/j.ijcard.2018.02.010. PMID: 29429637.
Article5. Brennan MA, Cookson BT. 2000; Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 38:31–40. DOI: 10.1046/j.1365-2958.2000.02103.x. PMID: 11029688.
Article6. Mouasni S, Gonzalez V, Schmitt A, Bennana E, Guillonneau F, Mistou S, Avouac J, Ea HK, Devauchelle V, Gottenberg JE, Chiocchia G, Tourneur L. 2019; The classical NLRP3 inflammasome controls FADD unconventional secretion through microvesicle shedding. Cell Death Dis. 10:190. DOI: 10.1038/s41419-019-1412-9. PMID: 30804327. PMCID: PMC6389912.
Article7. Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. 2016; Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 535:111–116. Erratum in: Nature. 2016;540:150. DOI: 10.1038/nature18590. PMID: 27281216.
Article8. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. 2015; Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 526:660–665. DOI: 10.1038/nature15514. PMID: 26375003.
Article9. Takeuchi O, Akira S. 2010; Pattern recognition receptors and inflammation. Cell. 140:805–820. DOI: 10.1016/j.cell.2010.01.022. PMID: 20303872.
Article10. Han Y, Liao X, Gao Z, Yang S, Chen C, Liu Y, Wang WE, Wu G, Chen X, Jose PA, Zhang Y, Zeng C. 2016; Cardiac troponin I exacerbates myocardial ischaemia/reperfusion injury by inducing the adhesion of monocytes to vascular endothelial cells via a TLR4/NF-κB-dependent pathway. Clin Sci (Lond). 130:2279–2293. DOI: 10.1042/CS20160373. PMID: 27682003.
Article11. Wang Q, Lin P, Li P, Feng L, Ren Q, Xie X, Xu J. 2017; Ghrelin protects the heart against ischemia/reperfusion injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci. 186:50–58. DOI: 10.1016/j.lfs.2017.08.004. PMID: 28782532.
Article12. Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J. 2018; Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem. 47:1497–1508. DOI: 10.1159/000490866. PMID: 29940584.
Article13. Gu Y, Liang Z, Wang H, Jin J, Zhang S, Xue S, Chen J, He H, Duan K, Wang J, Chang X, Qiu C. 2016; Tanshinone IIA protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation. Exp Ther Med. 12:1147–1152. DOI: 10.3892/etm.2016.3400. PMID: 27446335. PMCID: PMC4950596.
Article14. Cheng TO. 2007; Cardiovascular effects of Danshen. Int J Cardiol. 121:9–22. DOI: 10.1016/j.ijcard.2007.01.004. PMID: 17363091.
Article15. Li Q, Shen L, Wang Z, Jiang HP, Liu LX. 2016; Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 84:106–114. DOI: 10.1016/j.biopha.2016.09.014. PMID: 27643552.
Article16. Li L, Li DH, Qu N, Wen WM, Huang WQ. 2010; The role of ERK1/2 signaling pathway in coronary microembolization-induced rat myocardial inflammation and injury. Cardiology. 117:207–215. DOI: 10.1159/000321713. PMID: 21150201.
Article17. Su Q, Lv X, Sun Y, Ye Z, Kong B, Qin Z. 2018; Role of TLR4/MyD88/NF-κB signaling pathway in coronary microembolization-induced myocardial injury prevented and treated with nicorandil. Biomed Pharmacother. 106:776–784. DOI: 10.1016/j.biopha.2018.07.014. PMID: 29990871.
Article18. Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G. 2000; Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 279:H2587–H2592. DOI: 10.1152/ajpheart.2000.279.6.H2587. PMID: 11087208.
Article19. Liu T, Zhou Y, Wang JY, Su Q, Tang ZL, Liu YC, Li L. 2016; Coronary microembolization induces cardiomyocyte apoptosis in swine by activating the LOX-1-dependent mitochondrial pathway and caspase-8-dependent pathway. J Cardiovasc Pharmacol Ther. 21:209–218. DOI: 10.1177/1074248415599265. PMID: 26275408.
Article20. Wang JY, Chen H, Su X, Zhou Y, Li L. 2017; Atorvastatin pretreatment inhibits myocardial inflammation and apoptosis in swine after coronary microembolization. J Cardiovasc Pharmacol Ther. 22:189–195. DOI: 10.1177/1074248416662348. PMID: 27587240.
Article21. Bergsbaken T, Fink SL, Cookson BT. 2009; Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 7:99–109. DOI: 10.1038/nrmicro2070. PMID: 19148178. PMCID: PMC2910423.
Article22. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. 2016; Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 535:153–158. DOI: 10.1038/nature18629. PMID: 27383986. PMCID: PMC5539988.
Article23. Li H, Wang X, Xu A. 2018; Effect of paclitaxel+hirudin on the TLR4-MyD88 signaling pathway during inflammatory activation of human coronary artery smooth muscle cells and mechanistic analysis. Cell Physiol Biochem. 50:1301–1317. DOI: 10.1159/000494588. PMID: 30355931.
Article24. Michaeli A, Mezan S, Kühbacher A, Finkelmeier D, Elias M, Zatsepin M, Reed SG, Duthie MS, Rupp S, Lerner I, Burger-Kentischer A. 2018; Computationally designed bispecific MD2/CD14 binding peptides show TLR4 agonist activity. J Immunol. 201:3383–3391. DOI: 10.4049/jimmunol.1800380. PMID: 30348734.
Article25. Jiang Q, Yi M, Guo Q, Wang C, Wang H, Meng S, Liu C, Fu Y, Ji H, Chen T. 2015; Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-κB pathway. Int Immunopharmacol. 29:370–376. DOI: 10.1016/j.intimp.2015.10.027. PMID: 26507165.
Article26. Shih RH, Wang CY, Yang CM. 2015; NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 8:77. DOI: 10.3389/fnmol.2015.00077. PMID: 26733801. PMCID: PMC4683208.
Article27. Hu Y, Li Q, Pan Y, Xu L. 2019; Sal B alleviates myocardial ischemic injury by inhibiting TLR4 and the priming phase of NLRP3 inflammasome. Molecules. 24:4416. DOI: 10.3390/molecules24234416. PMID: 31816891. PMCID: PMC6930479.
Article28. Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, Duan J. 2017; The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed Pharmacother. 91:1042–1052. DOI: 10.1016/j.biopha.2017.05.033. PMID: 28525945.
Article29. Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. 2012; Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 220:3–10. Erratum in: Atherosclerosis. 2012;221:604. DOI: 10.1016/j.atherosclerosis.2012.01.014. PMID: 21774934.
Article30. Zhang X, Wang Q, Wang X, Chen X, Shao M, Zhang Q, Guo D, Wu Y, Li C, Wang W, Wang Y. 2019; Tanshinone IIA protects against heart failure post-myocardial infarction via AMPKs/mTOR-dependent autophagy pathway. Biomed Pharmacother. 112:108599. DOI: 10.1016/j.biopha.2019.108599. PMID: 30798134.
Article31. Zhang MQ, Zheng YL, Chen H, Tu JF, Shen Y, Guo JP, Yang XH, Yuan SR, Chen LZ, Chai JJ, Lu JH, Zhai CL. 2013; Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin. 34:1386–1396. DOI: 10.1038/aps.2013.91. PMID: 24077633. PMCID: PMC4006464.
Article32. Zhang Y, Zhang L, Chu W, Wang B, Zhang J, Zhao M, Li X, Li B, Lu Y, Yang B, Shan H. 2010; Tanshinone IIA inhibits miR-1 expression through p38 MAPK signal pathway in post-infarction rat cardiomyocytes. Cell Physiol Biochem. 26:991–998. DOI: 10.1159/000324012. PMID: 21220930.
Article33. Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A. 2018; Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 115:E10888–E10897. DOI: 10.1073/pnas.1809548115. PMID: 30381458. PMCID: PMC6243247.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resveratrol pretreatment alleviates NLRP3 inflammasomemediated cardiomyocyte pyroptosis by targeting TLR4/MyD88/ NF-κB signaling cascade in coronary microembolization-induced myocardial damage
- The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy
- Triptolide improves myocardial fibrosis in rats through inhibition of nuclear factor kappa B and NLR family pyrin domain containing 3 inflammasome pathway
- Overexpression of miR-146b-5p Ameliorates Neonatal Hypoxic Ischemic Encephalopathy by Inhibiting IRAK1/TRAF6/TAK1/NF-αB Signaling
- Chronic cold stress-induced myocardial injury: effects on oxidative stress, inflammation and pyroptosis