Brain Tumor Res Treat.
2022 Jul;10(3):195-199. 10.14791/btrt.2022.0021.
Cerebral Microangiopathy Mimicking a High-Grade Glioma in Old Age: A Case Report
- Affiliations
-
- 1Departments of Neurosurgery, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- 2Departments of Pathology, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- KMID: 2532252
- DOI: http://doi.org/10.14791/btrt.2022.0021
Abstract
- Cerebral microangiopathy (CM) has become a common disease related to improved neuroimaging modalities and an increased life expectancy. Intracerebral tumor-like mass lesions have rarely been reported in cases of cerebral amyloid angiopathy (CAA) in elderly patients. However, tumor-like mass lesions from CM without amyloid deposits have rarely been reported. These two angiopathies may have different pathogeneses and neuroimaging characteristics. Herein, we present the case of an 83-year-old man with CM mimicking a high-grade glioma. We described the possible pathogenesis and different neuroimaging features of CM compared to CAA.
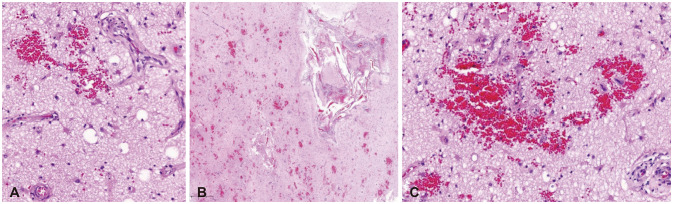
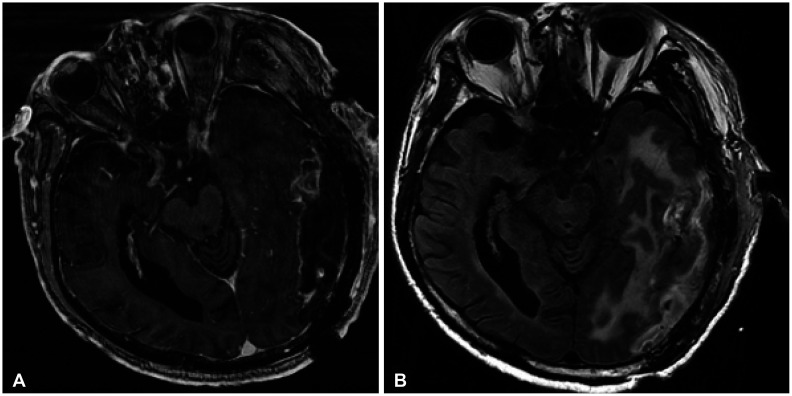
Figure
Reference
-
1. Okroglic S, Widmann CN, Urbach H, Scheltens P, Heneka MT. Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS One. 2013; 8:e53455. PMID: 23393549.2. Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011; 82:126–135. PMID: 20935330.3. Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke. 2011; 6:47–59. PMID: 21205241.4. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010; 9:689–701. PMID: 20610345.5. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019; 92:1146–1156. PMID: 31142635.6. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021; 18:170–186. PMID: 33293629.7. Nam KW, Kwon HM, Lim JS, Han MK, Nam H, Lee YS. The presence and severity of cerebral small vessel disease increases the frequency of stroke in a cohort of patients with large artery occlusive disease. PLoS One. 2017; 12:e0184944. PMID: 28991905.8. Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016; 1862:860–868. PMID: 26704177.9. Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010; 41(10 Suppl):S103–S106. PMID: 20876479.10. Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry. 2007; 78:702–706. PMID: 17210627.11. Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017; 88:669–674. PMID: 28600443.12. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017; 96:17–42. PMID: 28957666.13. Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JF, Jeukens CR, et al. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017; 88:426–432. PMID: 28031395.14. Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond). 2017; 131:2257–2274. PMID: 28798076.15. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013; 12:483–497. PMID: 23602162.16. Mikael LR, Paiva AMG, Gomes MM, Sousa ALL, Jardim PCBV, Vitorino PVO, et al. Vascular aging and arterial stiffness. Arq Bras Cardiol. 2017; 109:253–258. PMID: 28678931.17. Greenberg SM, William M. Feinberg award for excellence in clinical stroke: big pictures and small vessels. Stroke. 2017; 48:2628–2631. PMID: 28698255.18. Silek H, Erbas B. Cerebral amyloid angiopathy related inflammation presenting as steroid responsive brain mass. Turk Neurosurg. 2020; 30:629–631. PMID: 30649812.19. Kotsenas AL, Morris JM, Wald JT, Parisi JE, Campeau NG. Tumefactive cerebral amyloid angiopathy mimicking CNS neoplasm. AJR Am J Roentgenol. 2013; 200:50–56. PMID: 23255741.20. Inoue Y, Miyashita F, Minematsu K, Toyoda K. Clinical characteristics and outcomes of intracerebral hemorrhage in very elderly. J Stroke Cerebrovasc Dis. 2018; 27:97–102. PMID: 28893575.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nasal Cerebral Heterotopia-so called Nasal Glioma: A case report
- Spontaneous Regression of Glioma–Mimicking Brainstem Lesion in a Child: A Case Report
- Radiation-Induced Glioma In a Child
- Intracranial Undifferentiated Sarcoma Arising from a Low-Grade Glioma: A Case Report and Literature Review
- Thrombosed Large Middle Cerebral Artery Aneurysm Mimicking an Intra-Axial Brain Tumor: Case Report and Review of Literature