Brain Tumor Res Treat.
2024 Jan;12(1):58-62. 10.14791/btrt.2023.0039.
Spontaneous Regression of Glioma–Mimicking Brainstem Lesion in a Child: A Case Report
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea
- 2Center for Pediatric Cancer, National Cancer Center, Goyang, Korea
- 3Department of Neurology, National Cancer Center, Goyang, Korea
- 4Department of Cancer Control, National Cancer Center, Graduate School of Cancer Science and Policy, Goyang, Korea
- KMID: 2552339
- DOI: http://doi.org/10.14791/btrt.2023.0039
Abstract
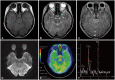
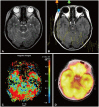
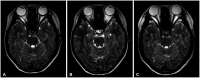
- Differential diagnosis of focal brainstem lesions detected on MRI is challenging, especially in young children. Formerly, brainstem gliomas were classified mainly based on MRI features and location. However, since 2016, the World Health Organization’s brainstem lesion classification requires tissue biopsy to reveal molecular characteristics. Although modern techniques of stereotactic or navigation-guided biopsy ensure accurate biopsy of the lesion with safety, biopsy of brainstem lesions is still generally not performed. Here, we report a focal brainstem lesion mimicking brainstem glioma in a 9-year-old girl. Initial MRI, MR spectroscopy, and 11 C-methionine positron emission tomography (PET) features suggested low-grade glioma or diffuse intrinsic pontine glioma. However, repeated MR spectroscopy, perfusion MRI, and 18 fluorodeoxyglucose PET findings suggested that it was more likely a non-tumorous lesion. As the patient presented not with a neurological manifestation but with precocious puberty, the attending oncologist chose to observe with regular follow-up MRI. The pontine lesion with high signal intensity on T2-weighted MRI regressed from the 6-month follow-up and became invisible on the 1.5-year follow-up MRI. We reviewed brainstem glioma–mimicking lesions in the literature and discussed the key points of differential diagnosis.
Figure
Reference
-
1. Green AL, Kieran MW. Pediatric brainstem gliomas: new understanding leads to potential new treatments for two very different tumors. Curr Oncol Rep. 2015; 17:436. PMID: 25702179.
Article2. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020; 22(12 Suppl 2):iv1–iv96. PMID: 33123732.
Article3. Ozair A, Khan E, Bhat V, Faruqi A, Nanda A. Pediatric brain tumors: from modern classification system to current principles of management. Turner SG, editor. Central nervous system tumors. London: IntechOpen;2021. p. 9–36.4. Leach JL, Roebker J, Schafer A, Baugh J, Chaney B, Fuller C, et al. MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the international DIPG registry. Neuro Oncol. 2020; 22:1647–1657. PMID: 32506137.
Article5. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.
Article6. Pincus DW, Richter EO, Yachnis AT, Bennett J, Bhatti MT, Smith A. Brainstem stereotactic biopsy sampling in children. J Neurosurg. 2006; 104(2 Suppl):108–114. PMID: 16506498.
Article7. Pérez-Gómez JL, Rodríguez-Alvarez CA, Marhx-Bracho A, Rueda-Franco F. Stereotactic biopsy for brainstem tumors in pediatric patients. Childs Nerv Syst. 2010; 26:29–34. PMID: 19784659.8. Mahdi J, Shah AC, Sato A, Morris SM, McKinstry RC, Listernick R, et al. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology. 2017; 88:1584–1589. PMID: 28330960.
Article9. Culleton S, McKenna B, Dixon L, Taranath A, Oztekin O, Prasad C, et al. Imaging pitfalls in paediatric posterior fossa neoplastic and non-neoplastic lesions. Clin Radiol. 2021; 76:391.e19–391.e31.
Article10. Huisman TA. Tumor-like lesions of the brain. Cancer Imaging. 2009; 9:S10–S13. PMID: 19965288.
Article11. Go JL, Acharya J, Rajamohan AG. Is it or is it not? Brain tumor mimics. Semin Roentgenol. 2018; 53:62–76. PMID: 29405957.
Article12. Cianfoni A, Niku S, Imbesi SG. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. AJNR Am J Neuroradiol. 2007; 28:272–277. PMID: 17296993.13. Tomura N, Saginoya T, Kaneko C. 18F-fluorodeoxy glucose and 11C-methionine accumulation in demyelinating lesions. World J Nucl Med. 2022; 21:261–266. PMID: 36398309.
Article14. Kawase Y, Yamamoto Y, Kameyama R, Kawai N, Kudomi N, Nishiyama Y. Comparison of 11C-methionine PET and 18F-FDG PET in patients with primary central nervous system lymphoma. Mol Imaging Biol. 2011; 13:1284–1289. PMID: 21042866.
Article15. Frazier JL, Lee J, Thomale UW, Noggle JC, Cohen KJ, Jallo GI. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr. 2009; 3:259–269. PMID: 19338403.
Article16. Gwak HS, Park HJ. Developing chemotherapy for diffuse pontine intrinsic gliomas (DIPG). Crit Rev Oncol Hematol. 2017; 120:111–119. PMID: 29198324.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Treatment With Radiotherapy and Immunotherapy for Isocitrate Dehydrogenase Mutant Brainstem Glioma in Adult: A Case Report
- Clinical observation on brainstem glioma in childhood
- A Case of Brainstem Encephalitis
- Primary central nervous system lymphoma in the brainstem and cervical spinal cord: a case report and literature review
- Developmental Venous Anomalies of the Brainstem Associated with Spontaneous Vertigo: A Case Report