Intest Res.
2022 Jul;20(3):303-312. 10.5217/ir.2021.00018.
Factors associated with anti-tumor necrosis factor effectiveness to prevent postoperative recurrence in Crohn’s disease
- Affiliations
-
- 1Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL, USA
- 2Inflammatory Bowel Unit, Inserm, 3iHP, CHU Clermont-Ferrand, Clermont Auvergne University, Clermont-Ferrand, France
- 3USC INRae 2018, M2iSH, Inserm U1071, 3iHP, Clermont Auvergne University, Clermont-Ferrand, France
- 4Department of Surgery, University of Chicago Medicine, Chicago, IL, USA
- 5Biostatistics Unit, DRCI, CHU Clermont-Ferrand, Clermont Auvergne University, Clermont-Ferrand, France
- KMID: 2531984
- DOI: http://doi.org/10.5217/ir.2021.00018
Abstract
- Background/Aims
We assessed the effectiveness of anti-TNF agents and its associated factors to prevent endoscopic and clinical postoperative recurrence (POR) in Crohn’s disease (CD).
Methods
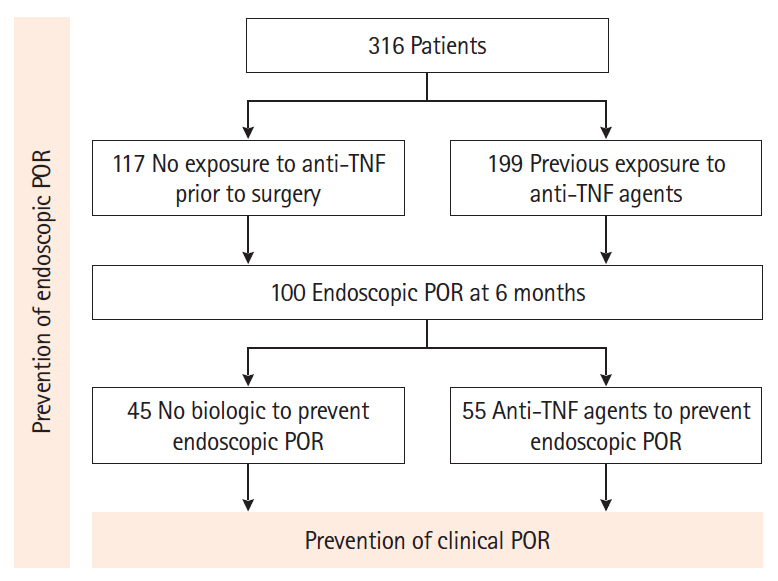
From a prospectively-maintained database, we retrieved 316 CD patients who underwent intestinal resection (2011–2017). Endoscopic (Rutgeerts index ≥ i2 at 6 months) and clinical (recurrence of symptoms leading to hospitalization or therapeutic escalation) POR were assessed.
Results
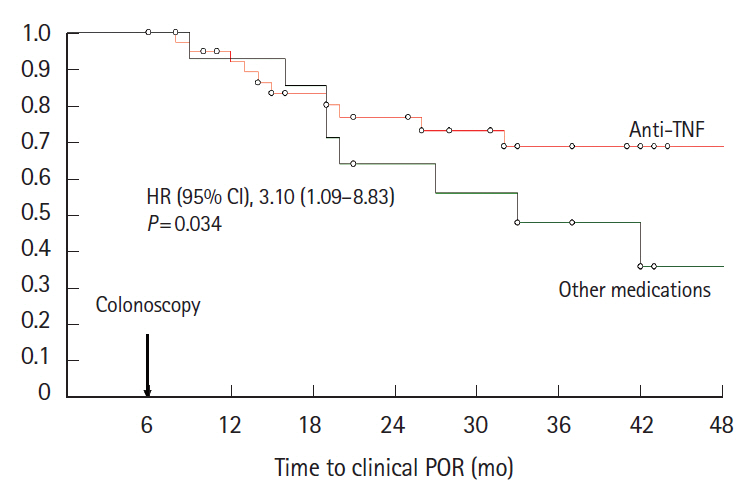
In 117 anti-TNF-naïve patients, anti-TNF therapy was more effective than immunosuppressive agents (odds ratio [OR], 8.8; 95% confidence interval [CI], 1.8–43.9; P= 0.008) and no medication/5-aminosalicylates (OR, 5.2; 95% CI, 1.0–27.9; P= 0.05) to prevent endoscopic POR. In 199 patients exposed to anti-TNF prior to the surgery, combination with anti-TNF and immunosuppressive agents was more effective than anti-TNF monotherapy (OR, 2.32; 95% CI, 1.02–5.31; P= 0.046) to prevent endoscopic POR. Primary failure to anti-TNF agent prior to surgery was predictive of anti-TNF failure to prevent endoscopic POR (OR, 2.41; 95% CI, 1.10–5.32; P= 0.03). When endoscopic POR despite anti-TNF prophylactic medication (n = 55), optimizing anti-TNF and adding an immunosuppressive drug was the most effective option to prevent clinical POR (hazard ratio, 7.38; 95% CI, 1.54–35.30; P= 0.012). Anti-TNF therapy was the best option to prevent clinical POR (hazard ratio, 3.10; 95% CI, 1.09–8.83; P= 0.034) in patients with endoscopic POR who did not receive any biologic to prevent endoscopic POR (n = 55).
Conclusions
Anti-TNF was the most effective medication to prevent endoscopic and clinical POR. Combination with anti-TNF and immunosuppressive agents should be considered in patients previously exposed to anti-TNF.
Figure
Cited by 1 articles
-
Prevention of postoperative recurrence in Crohn’s disease: the never-ending story
Jung-Bin Park, Sang Hyoung Park
Intest Res. 2022;20(3):279-280. doi: 10.5217/ir.2022.00081.
Reference
-
1. Pariente B, Mary JY, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015; 148:52–63.
Article2. Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012; 61:241–247.
Article3. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010; 105:289–297.
Article4. Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012; 35:625–633.
Article5. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990; 99:956–963.
Article6. Buisson A, Cannon L, Umanskiy K, et al. Natural history of Crohn’s disease postoperative recurrence in a US Referral Center in the era of biologics and therapeutic intensification based on early endoscopic findings. Gastroenterology. 2018; 154:S1–S128.7. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015; 385:1406–1417.
Article8. Buisson A, Chevaux JB, Bommelaer G, Peyrin-Biroulet L. Diagnosis, prevention and treatment of postoperative Crohn’s disease recurrence. Dig Liver Dis. 2012; 44:453–460.
Article9. Carla-Moreau A, Paul S, Roblin X, Genin C, Peyrin-Biroulet L. Prevention and treatment of postoperative Crohn’s disease recurrence with anti-TNF therapy: a meta-analysis of controlled trials. Dig Liver Dis. 2015; 47:191–196.
Article10. Gionchetti P, Dignass A, Danese S, et al. 3rd European evidencebased consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis. 2017; 11:135–149.
Article11. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009; 136:441–450.
Article12. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology. 2016; 150:1568–1578.13. De Cruz P, Kamm MA, Hamilton AL, et al. Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients: a POCER study analysis. Aliment Pharmacol Ther. 2015; 42:867–879.
Article14. Savarino E, Bodini G, Dulbecco P, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn’s disease: a randomized controlled trial. Am J Gastroenterol. 2013; 108:1731–1742.
Article15. Boucher AL, Pereira B, Decousus S, et al. Endoscopy-based management decreases the risk of postoperative recurrences in Crohn’s disease. World J Gastroenterol. 2016; 22:5068–5078.
Article16. De Cruz P, Bernardi MP, Kamm MA, et al. Postoperative recurrence of Crohn’s disease: impact of endoscopic monitoring and treatment step-up. Colorectal Dis. 2013; 15:187–197.
Article17. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.
Article18. Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV Jr. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015; 148:64–76.
Article19. Burr NE, Hall B, Hamlin PJ, Selinger CP, Ford AC, O’Connor A. Systematic review and network meta-analysis of medical therapies to prevent recurrence of post-operative Crohn’s disease. J Crohns Colitis. 2019; 13:693–701.
Article20. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010; 362:1383–1395.
Article21. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis. 2016; 10:1259–1266.
Article22. Reenaers C, Louis E, Belaiche J, Seidel L, Keshav S, Travis S. Does co-treatment with immunosuppressors improve outcome in patients with Crohn’s disease treated with adalimumab? Aliment Pharmacol Ther. 2012; 36:1040–1048.
Article23. Kennedy NA, Heap GA, Green HD, et al. Predictors of antiTNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019; 4:341–353.24. Roblin X, Vérot C, Paul S, et al. Is the pharmacokinetic profile of a first anti-TNF predictive of the clinical outcome and pharmacokinetics of a second anti-TNF? Inflamm Bowel Dis. 2018; 24:2078–2085.
Article25. Fay S, Ungar B, Paul S, et al. The association between drug levels and endoscopic recurrence in postoperative patients with Crohn’s disease treated with tumor necrosis factor inhibitors. Inflamm Bowel Dis. 2017; 23:1924–1929.
Article26. Wright EK, Kamm MA, De Cruz P, et al. Anti-TNF therapeutic drug monitoring in postoperative Crohn’s disease. J Crohns Colitis. 2018; 12:653–661.
Article27. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015; 41:613–623.
Article28. Yamada A, Komaki Y, Patel N, et al. The use of vedolizumab in preventing postoperative recurrence of Crohn’s disease. Inflamm Bowel Dis. 2018; 24:502–509.
Article29. Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn’s disease: a prospective pilot study. Inflamm Bowel Dis. 2009; 15:1460–1466.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preoperative use of anti-tumor necrosis factor therapy in Crohn's disease: promises and pitfalls
- Recurrence of Tuberculosis after Resuming a TNF-Inhibitor in a Patient with Crohn's Disease
- Biological Therapy for Inflammatory Bowel Disease in Children
- Anti-tumor Necrosis Factor Therapy for Crohn Disease: Friend or Foe to the Surgeon?
- Biological Therapy for the Prevention and Treatment of Postoperative Endoscopic Recurrence in Crohn's Disease: Time for Acceptance?