Clin Endosc.
2022 Jul;55(4):549-557. 10.5946/ce.2021.227.
Comparison of tube-assisted mapping biopsy with digital single-operator peroral cholangioscopy for preoperative evaluation of biliary tract cancer
- Affiliations
-
- 1Department of Hepato-Biliary-Pancreatic Medicine, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan
- KMID: 2531949
- DOI: http://doi.org/10.5946/ce.2021.227
Abstract
- Background/Aims
Digital single-operator cholangioscopy (DSOC)-guided mapping biopsy (DMB) and tube-assisted mapping biopsy (TMB) are two techniques used for preoperative evaluation of biliary tract cancer (BTC). However, data regarding the diagnostic performance of these techniques are limited.
Methods
We retrospectively examined consecutive patients with BTC who underwent either technique at our institution between 2018 and 2020. We evaluated the technical success rate, adequate tissue acquisition rate, and diagnostic performance of these techniques for the evaluation of lateral spread of BTC.
Results
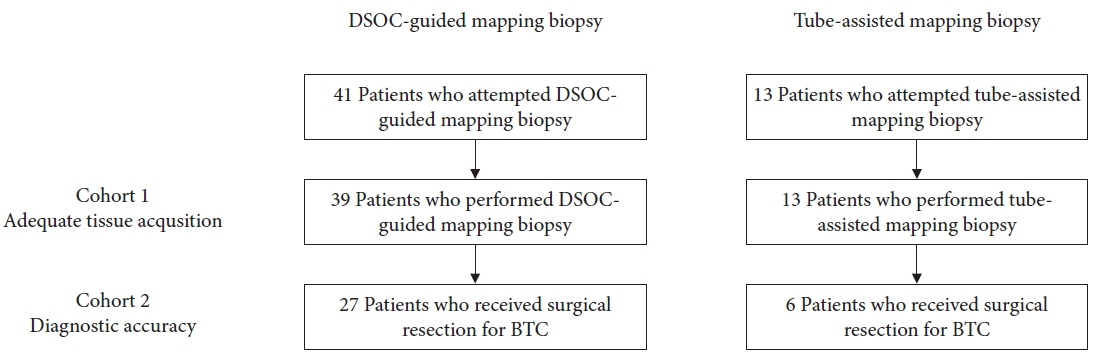
A total of 54 patients were included in the study. The technical success rate of reaching the target sites was 95% for DMB and 100% for TMB. The adequate tissue acquisition rate was 61% for DMB and 69% for TMB. The adequate tissue acquisition rate was low, especially for target sites beyond the secondary biliary radicles. The sensitivity of DMB alone was 39%, which improved to 65% when combined with visual impression. Experts demonstrated a higher negative predictive value and diagnostic accuracy with respect to both DSOC visual impression and DMB for the evaluation of lateral spread of BTC compared to trainees.
Conclusions
Adequate tissue acquisition rates were similar between the two techniques. Since DMB requires expertise, TMB may be an acceptable option when DSOC is unavailable or when DSOC expertise is limited.
Keyword
Figure
Reference
-
1. Igami T, Nagino M, Oda K, et al. Clinicopathologic study of cholangiocarcinoma with superficial spread. Ann Surg. 2009; 249:296–302.2. Mohamadnejad M, DeWitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011; 73:71–78.3. Ito K, Sakamoto Y, Isayama H, et al. The impact of MDCT and endoscopic transpapillary mapping biopsy to predict longitudinal spread of extrahepatic cholangiocarcinoma. J Gastrointest Surg. 2018; 22:1528–1537.4. Watadani T, Akahane M, Yoshikawa T, et al. Preoperative assessment of hilar cholangiocarcinoma using multidetector-row CT: correlation with histopathological findings. Radiat Med. 2008; 26:402–407.5. Park HS, Lee JM, Choi JY, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol. 2008; 190:396–405.6. Noda Y, Fujita N, Kobayashi G, et al. Intraductal ultrasonography before biliary drainage and transpapillary biopsy in assessment of the longitudinal extent of bile duct cancer. Dig Endosc. 2008; 20:73–78.7. Kawakami H, Kuwatani M, Abe Y, et al. A guidewire-assisted biopsy technique to assist advancement through a biliary stricture to perform selective mapping biopsy. Endoscopy. 2015; 47 Suppl 1 UCTN:E217–E218.8. Hijioka S, Hara K, Mizuno N, et al. A novel technique for endoscopic transpapillary “mapping biopsy specimens” of superficial intraductal spread of bile duct carcinoma (with videos). Gastrointest Endosc. 2014; 79:1020–1025.9. Hamada T, Takahara N, Nakai Y, et al. The “zipline” technique for endoscopic transpapillary biliary biopsy. Endoscopy. 2020; 52:236–237.10. Manta R, Frazzoni M, Conigliaro R, et al. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc. 2013; 27:1569–1572.11. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012; 75:347–353.12. Gerges C, Beyna T, Tang RS, et al. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: a prospective, randomized, multicenter trial (with video). Gastrointest Endosc. 2020; 91:1105–1113.13. Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015; 82:608–614.e2.14. Onoyama T, Hamamoto W, Sakamoto Y, et al. Peroral cholangioscopy-guided forceps mapping biopsy for evaluation of the lateral extension of biliary tract cancer. J Clin Med. 2021; 10:597.15. Ogawa T, Kanno Y, Koshita S, et al. Cholangioscopy- versus fluoroscopy-guided transpapillary mapping biopsy for preoperative evaluation of extrahepatic cholangiocarcinoma: a prospective randomized crossover study. Surg Endosc. 2021; 35:6481–6488.16. Jang S, Stevens T, Kou L, et al. Efficacy of digital single-operator cholangioscopy and factors affecting its accuracy in the evaluation of indeterminate biliary stricture. Gastrointest Endosc. 2020; 91:385–393.e1.17. Ko SW, Lee SS, So H, et al. A novel method of biopsy for indeterminate pancreaticobiliary strictures: tube-assisted biopsy. Endoscopy. 2020; 52:589–594.18. Okada H, Uza N, Matsumori T, et al. A novel technique for mapping biopsy of bile duct cancer. Endoscopy. 2021; 53:647–651.19. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.20. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48:452–458.21. Kulpatcharapong S, Pittayanon R, J Kerr S, et al. Diagnostic performance of different cholangioscopes in patients with biliary strictures: a systematic review. Endoscopy. 2020; 52:174–185.22. Kanno Y, Koshita S, Ogawa T, et al. Peroral cholangioscopy by SpyGlass DS versus CHF-B260 for evaluation of the lateral spread of extrahepatic cholangiocarcinoma. Endosc Int Open. 2018; 6:E1349–E1354.23. Nishikawa T, Tsuyuguchi T, Sakai Y, et al. Preoperative assessment of longitudinal extension of cholangiocarcinoma with peroral video-cholangioscopy: a prospective study. Dig Endosc. 2014; 26:450–457.24. Onoyama T, Takeda Y, Kawata S, et al. Adequate tissue acquisition rate of peroral cholangioscopy-guided forceps biopsy. Ann Transl Med. 2020; 8:1073.25. Osanai M, Itoi T, Igarashi Y, et al. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: a prospective multicenter study. Endoscopy. 2013; 45:635–642.26. Ogawa T, Ito K, Koshita S, et al. Usefulness of cholangioscopic-guided mapping biopsy using SpyGlass DS for preoperative evaluation of extrahepatic cholangiocarcinoma: a pilot study. Endosc Int Open. 2018; 6:E199–E204.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- When the Going Gets Tough: Utilization of SpyGlass, or Drainage Followed by Stone Removal
- The Role of Peroral Cholangioscopy in Evaluating Indeterminate Biliary Strictures
- Recent update of therapeutic application of peroral cholangioscopy
- Minimally Invasive Approach Using Digital Single-Operator Peroral Cholangioscopy-Guided Electrohydraulic Lithotripsy and Endoscopic Nasogallbladder Drainage for the Management of High-Grade Mirizzi Syndrome
- Advanced Imaging Technology in Biliary Tract Diseases:Narrow-Band Imaging of the Bile Duct