Acute Crit Care.
2022 May;37(2):209-216. 10.4266/acc.2021.00899.
Hearing screening outcomes in pediatric critical care survivors: a 1-year report
- Affiliations
-
- 1Department of Pediatrics, Faculty of Medicine, Thammasat University, Pathumthani, Thailand
- 2Division of Pediatric Critical Care, Department of Pediatrics, Thammasat University Hospital, Faculty of Medicine, Thammasat University, Pathumthani, Thailand
- KMID: 2531678
- DOI: http://doi.org/10.4266/acc.2021.00899
Abstract
- Background
Hearing loss is a potentially serious complication that can occur after surviving a critical illness. Study on screening for hearing problems in pediatric critical care survivors beyond the neonatal period is lacking. This study aimed to identify the prevalence of abnormal hearing screening outcomes using transitory evoked otoacoustic emission (TEOAE) screening in children who survived critical illness and to find possible associating factors for abnormal hearing screening results.
Methods
This study was a single-center, prospective, observational study. All children underwent otoscopy to exclude external and middle ear abnormalities before undergoing TEOAE screening. The screening was conducted before hospital discharge. Descriptive statistics, chi-square, and logistic regression tests were used for data analysis.
Results
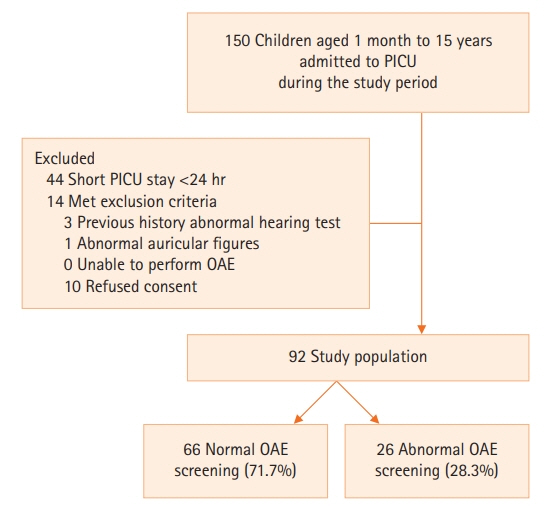
A total of 92 children were enrolled. Abnormal TEOAE responses were identified in 26 participants (28.3%). Children with abnormal responses were significantly younger than those with normal responses with a median age of 10.0 months and 43.5 months, respectively (P<0.001). Positive association with abnormal responses was found in children younger than 12 months of age (adjusted odds ratio [OR], 3.07; 95% confidence interval [CI], 1.06–8.90) and children with underlying genetic conditions (adjusted OR, 6.95; 95% CI, 1.49–32.54).
Conclusions
Our study demonstrates a high prevalence of abnormal TEOAE screening responses in children surviving critical illness, especially in patients younger than 12 months of age. More extensive studies should be performed to identify the prevalence and associated risk factors of hearing problems in critically ill children.
Keyword
Figure
Reference
-
1. Halpern NA, Pastores SM, Price JB, Alicea M. Hearing loss in critical care: an unappreciated phenomenon. Crit Care Med. 1999; 27:211–9.2. Guyton A, Hall J. Sense of hearing. In : Hall J, editor. Textbook of medical physiology. Philadelphia: W. B. Saunders;1996. p. 663–73.3. Fitzgerald DC. Head trauma: hearing loss and dizziness. J Trauma. 1996; 40:488–96.4. Guo Y, Wu Y, Chen W, Lin J. Endotoxic damage to the stria vascularis: the pathogenesis of sensorineural hearing loss secondary to otitis media? J Laryngol Otol. 1994; 108:310–3.
Article5. Zeeshan F, Bari A, Dugal MN, Saeed F. Hearing impairment after acute bacterial meningitis in children. Pak J Med Sci. 2018; 34:655–9.
Article6. Davis LE, Johnsson LG. Viral infections of the inner ear: clinical, virologic, and pathologic studies in humans and animals. Am J Otolaryngol. 1983; 4:347–62.7. Lerner SA, Matz GJ. Aminoglycoside ototoxicity. Am J Otolaryngol. 1980; 1:169–79.
Article8. Masur H. Antimicrobials. In : Chernow B, editor. The pharmacologic approach to the critically ill patient. Baltimore (ML): Williams & Wilkins;1994. p. 685–99.9. Brummett RE. Ototoxicity of vancomycin and analogues. Otolaryngol Clin North Am. 1993; 26:821–8.
Article10. Rybak LP. Ototoxicity of loop diuretics. Otolaryngol Clin North Am. 1993; 26:829–44.
Article11. Jung TT, Rhee CK, Lee CS, Park YS, Choi DC. Ototoxicity of salicylate, nonsteroidal antiinflammatory drugs, and quinine. Otolaryngol Clin North Am. 1993; 26:791–810.
Article12. Schaffartzik W, Hirsch J, Frickmann F, Kuhly P, Ernst A. Hearing loss after spinal and general anesthesia: a comparative study. Anesth Analg. 2000; 91:1466–72.
Article13. Neumann K, Indermark A. Validation of a new TEOAE-AABR device for newborn hearing screening. Int J Audiol. 2012; 51:570–5.
Article14. Yin L, Bottrell C, Clarke N, Shacks J, Poulsen MK. Otoacoustic emissions: a valid, efficient first-line hearing screen for preschool children. J Sch Health. 2009; 79:147–52.
Article15. Johnson JL, White KR, Widen JE, Gravel JS, James M, Kennalley T, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005; 116:663–72.
Article16. van Dommelen P, Mohangoo AD, Verkerk PH, van der Ploeg CP, van Straaten HL; Dutch NICU Neonatal Hearing Screening Working Group. Risk indicators for hearing loss in infants treated in different neonatal intensive care units. Acta Paediatr. 2010; 99:344–9.
Article17. van Dommelen P, Verkerk PH, van Straaten HL; Dutch Neonatal Intensive Care Unit Neonatal Hearing Screening Working Group. Hearing loss by week of gestation and birth weight in very preterm neonates. J Pediatr. 2015; 166:840–3.18. De Capua B, Costantini D, Martufi C, Latini G, Gentile M, De Felice C. Universal neonatal hearing screening: the Siena (Italy) experience on 19,700 newborns. Early Hum Dev. 2007; 83:601–6.
Article19. Eavey RD, Pinto LE, Thornton AR, Herrmann BS, do Carmo Bertero M, Saenz A. Early hearing testing of still critically ill neonates. Arch Otolaryngol Head Neck Surg. 1996; 122:289–93.
Article20. Cooper AC, Commers AR, Finkelstein M, Lipnik PG, Tollefson LM, Wilcox RA, et al. Otoacoustic emission screen results in critically ill neonates who received gentamicin in the first week of life. Pharmacotherapy. 2011; 31:649–57.
Article21. Hamill-Ruth RJ, Ruth RA, Googer K, Volles D, Deivert M, Turrentine B. Use of otoacoustic emissions to screen for hearing loss in critically ill patients. Audiology. 1998; 37:344–52.22. Krug E, Cieza A, Chadha S, Sminkey L, Martinez R, Stevens G, et al. Childhood hearing loss: strategies for prevention and care. Geneva: World Health Organization;2016.23. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996; 24:743–52.24. Belletti A, Lerose CC, Zangrillo A, Landoni G. Vasoactive-Inotropic Score: evolution, clinical utility, and pitfalls. J Cardiothorac Vasc Anesth. 2021; 35:3067–77.
Article25. Quirk WS, Seidman MD. Cochlear vascular changes in response to loud noise. Am J Otol. 1995; 16:322–5.26. Fligor BJ, Neault MW, Mullen CH, Feldman HA, Jones DT. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005; 115:1519–28.
Article27. Black RE, Lau WK, Weinstein RJ, Young LS, Hewitt WL. Ototoxicity of amikacin. Antimicrob Agents Chemother. 1976; 9:956–61.
Article28. So TY. Use of ototoxic medications in neonates-the need for follow-up hearing test. J Pediatr Pharmacol Ther. 2009; 14:200–3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Newborn Hearing Loss and Newborn Hearing Screening
- Post–intensive-care morbidity among pediatric patients in Thailand: prevalence, risk factors, and the importance of the post–intensive-care clinic
- Interfacility transport of critically ill children
- Pediatric postintensive care syndrome: high burden and a gap in evaluation tools for limited-resource settings
- Cut-Off Values of the Post-Intensive Care Syndrome Questionnaire for the Screening of Unplanned Hospital Readmission within One Year