Endocrinol Metab.
2022 Jun;37(3):408-414. 10.3803/EnM.2022.302.
Human Tissue-Engineered Skeletal Muscle: A Tool for Metabolic Research
- Affiliations
-
- 1Center for Advanced Bio-Molecular Recognition, Korea Institute of Science and Technology, Seoul, Korea
- 2Department of Biomedical Sciences, College of Medicine, Korea University, Seoul, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea
- KMID: 2531384
- DOI: http://doi.org/10.3803/EnM.2022.302
Abstract
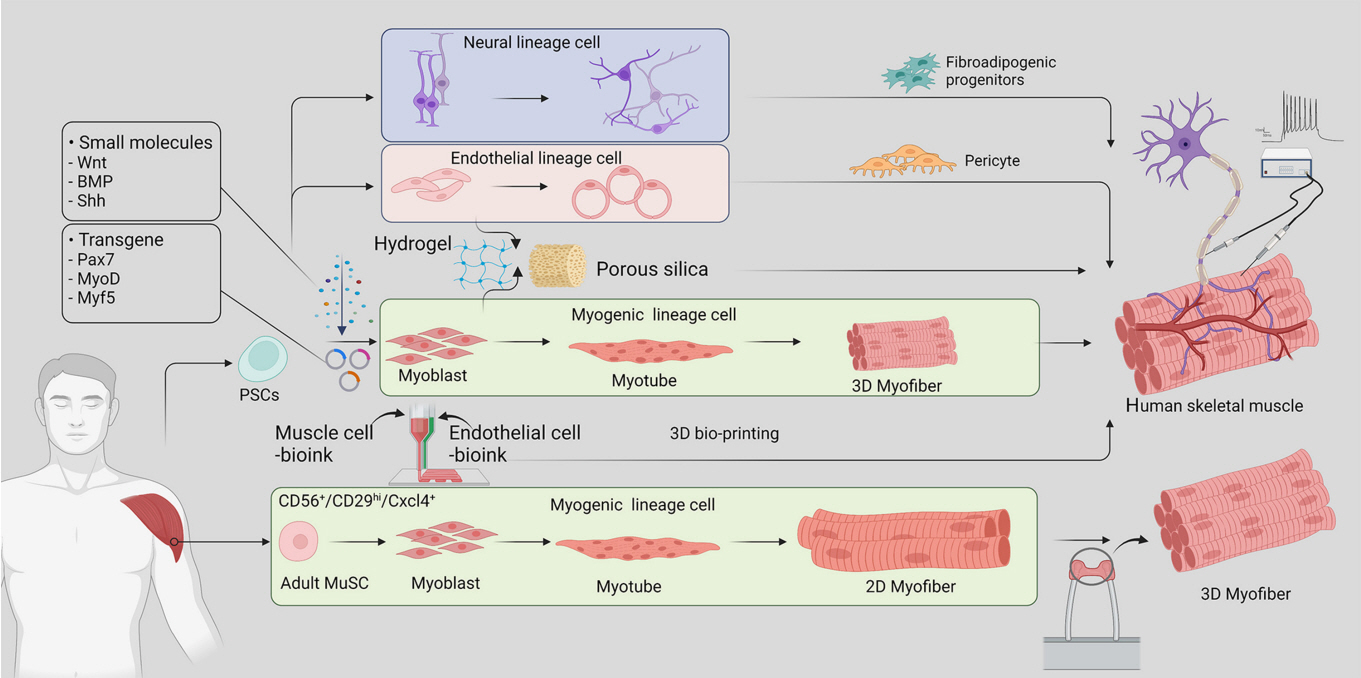
- Skeletal muscle is now regarded as an endocrine organ based on its secretion of myokines and exerkines, which, in response to metabolic stimuli, regulate the crosstalk between the skeletal muscle and other metabolic organs in terms of systemic energy homeostasis. This conceptual basis of skeletal muscle as a metabolically active organ has provided insights into the potential role of physical inactivity and conditions altering muscle quality and quantity in the development of multiple metabolic disorders, including insulin resistance, obesity, and diabetes. Therefore, it is important to understand human muscle physiology more deeply in relation to the pathophysiology of metabolic diseases. Since monolayer cell lines or animal models used in conventional research differ from the pathophysiological features of the human body, there is increasing need for more physiologically relevant in vitro models of human skeletal muscle. Here, we introduce recent studies on in vitro models of human skeletal muscle generated from adult myogenic progenitors or pluripotent stem cells and summarize recent progress in the development of three-dimensional (3D) bioartificial muscle, which mimics the physiological complexity of native skeletal muscle tissue in terms of maturation and functionality. We then discuss the future of skeletal muscle 3D-organoid culture technology in the field of metabolic research for studying pathological mechanisms and developing personalized therapeutic strategies.
Keyword
Figure
Reference
-
1. Smith AG, Muscat GE. Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease. Int J Biochem Cell Biol. 2005; 37:2047–63.
Article2. Otero-Diaz B, Rodriguez-Flores M, Sanchez-Munoz V, Monraz-Preciado F, Ordonez-Ortega S, Becerril-Elias V, et al. Exercise induces white adipose tissue browning across the weight spectrum in humans. Front Physiol. 2018; 9:1781.3. Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015; 96:183–95.
Article4. Doncheva NT, Palasca O, Yarani R, Litman T, Anthon C, Groenen MA, et al. Human pathways in animal models: possibilities and limitations. Nucleic Acids Res. 2021; 49:1859–71.
Article5. Sanoh S, Horiguchi A, Sugihara K, Kotake Y, Tayama Y, Uramaru N, et al. Predictability of metabolism of ibuprofen and naproxen using chimeric mice with human hepatocytes. Drug Metab Dispos. 2012; 40:2267–72.
Article6. Varga O, Harangi M, Olsson IA, Hansen AK. Contribution of animal models to the understanding of the metabolic syndrome: a systematic overview. Obes Rev. 2010; 11:792–807.
Article7. Kafkafi N, Agassi J, Chesler EJ, Crabbe JC, Crusio WE, Eilam D, et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci Biobehav Rev. 2018; 87:218–32.
Article8. Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998; 2:559–69.
Article9. Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014; 10:131–45.
Article10. Cox TC. Utility and limitations of animal models for the functional validation of human sequence variants. Mol Genet Genomic Med. 2015; 3:375–82.
Article11. Xu X, Wilschut KJ, Kouklis G, Tian H, Hesse R, Garland C, et al. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Reports. 2015; 5:419–34.
Article12. Garcia SM, Tamaki S, Lee S, Wong A, Jose A, Dreux J, et al. High-yield purification, preservation, and serial transplantation of human satellite cells. Stem Cell Reports. 2018; 10:1160–74.
Article13. Jalal S, Dastidar S, Tedesco FS. Advanced models of human skeletal muscle differentiation, development and disease: three-dimensional cultures, organoids and beyond. Curr Opin Cell Biol. 2021; 73:92–104.
Article14. Afshar Bakooshli M, Lippmann ES, Mulcahy B, Iyer N, Nguyen CT, Tung K, et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife. 2019; 8:e44530.
Article15. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020; 21:571–84.
Article16. Moyle LA, Jacques E, Gilbert PM. Engineering the next generation of human skeletal muscle models: from cellular complexity to disease modeling. Curr Opin Biomed Eng. 2020; 16:9–18.
Article17. Osaki T, Uzel SG, Kamm RD. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci Adv. 2018; 4:eaat5847.
Article18. Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro-adipogenic progenitors cross-talk in skeletal muscle: the social network. Front Physiol. 2019; 10:1074.
Article19. Hernandez-Hernandez JM, Garcia-Gonzalez EG, Brun CE, Rudnicki MA. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol. 2017; 72:10–8.
Article20. Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012; 4:a008342.
Article21. Tajbakhsh S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med. 2009; 266:372–89.
Article22. Kim JH, Han GC, Seo JY, Park I, Park W, Jeong HW, et al. Sex hormones establish a reserve pool of adult muscle stem cells. Nat Cell Biol. 2016; 18:930–40.
Article23. Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010; 120:11–9.
Article24. Iberite F, Gruppioni E, Ricotti L. Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges. NPJ Regen Med. 2022; 7:23.
Article25. Maffioletti SM, Sarcar S, Henderson AB, Mannhardt I, Pinton L, Moyle LA, et al. Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep. 2018; 23:899–908.
Article26. Rao L, Qian Y, Khodabukus A, Ribar T, Bursac N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun. 2018; 9:126.
Article27. Batista TM, Jayavelu AK, Wewer Albrechtsen NJ, Iovino S, Lebastchi J, Pan H, et al. A cell-autonomous signature of dysregulated protein phosphorylation underlies muscle insulin resistance in type 2 diabetes. Cell Metab. 2020; 32:844–59.
Article28. Ebrahimi M, Lad H, Fusto A, Tiper Y, Datye A, Nguyen CT, et al. De novo revertant fiber formation and therapy testing in a 3D culture model of Duchenne muscular dystrophy skeletal muscle. Acta Biomater. 2021; 132:227–44.
Article29. Rajabian N, Shahini A, Asmani M, Vydiam K, Choudhury D, Nguyen T, et al. Bioengineered skeletal muscle as a model of muscle aging and regeneration. Tissue Eng Part A. 2021; 27:74–86.
Article30. Bersini S, Gilardi M, Ugolini GS, Sansoni V, Talo G, Perego S, et al. Engineering an environment for the study of fibrosis: a 3D human muscle model with endothelium specificity and endomysium. Cell Rep. 2018; 25:3858–68.
Article31. Choi YJ, Jun YJ, Kim DY, Yi HG, Chae SH, Kang J, et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. 2019; 206:160–9.
Article32. Wang J, Khodabukus A, Rao L, Vandusen K, Abutaleb N, Bursac N. Engineered skeletal muscles for disease modeling and drug discovery. Biomaterials. 2019; 221:119416.
Article33. Nawrocki AR, Scherer PE. The delicate balance between fat and muscle: adipokines in metabolic disease and musculoskeletal inflammation. Curr Opin Pharmacol. 2004; 4:281–9.
Article34. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017; 389:2239–51.
Article35. Teng S, Huang P. The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function. Stem Cell Res Ther. 2019; 10:103.
Article36. Faustino Martins JM, Fischer C, Urzi A, Vidal R, Kunz S, Ruffault PL, et al. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell. 2020; 27:498.
Article37. Iovino S, Burkart AM, Warren L, Patti ME, Kahn CR. Myotubes derived from human-induced pluripotent stem cells mirror in vivo insulin resistance. Proc Natl Acad Sci U S A. 2016; 113:1889–94.
Article38. Priest C, Tontonoz P. Inter-organ cross-talk in metabolic syndrome. Nat Metab. 2019; 1:1177–88.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glucose Uptake and Insulin Response in Tissue-engineered Human Skeletal Muscle
- Impact of Skeletal Muscle Mass on Metabolic Health
- The Expression of Cytokines and Chemokine mRNA by Human Skeletal Muscle Cell Line (SKM14)
- Isolation and Characterization of Human Muscle Cells
- Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia