Ann Pediatr Endocrinol Metab.
2022 Jun;27(2):83-89. 10.6065/apem.2244124.062.
Classic and backdoor pathways of androgen biosynthesis in human sexual development
- Affiliations
-
- 1Department of Pediatrics, Chonnam National University Medical School & Children’s Hospital, Gwangju, Korea
- KMID: 2531271
- DOI: http://doi.org/10.6065/apem.2244124.062
Abstract
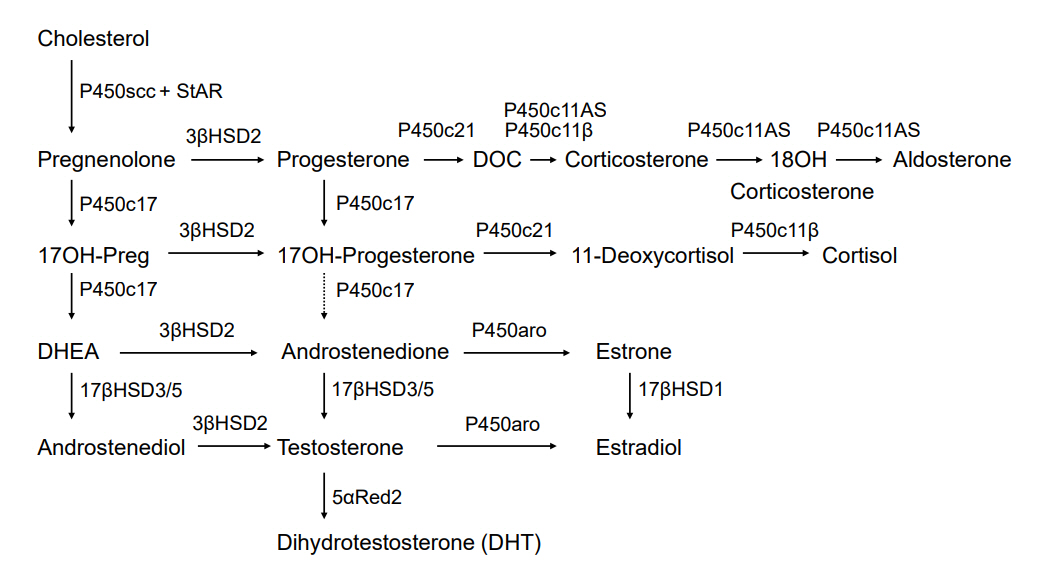
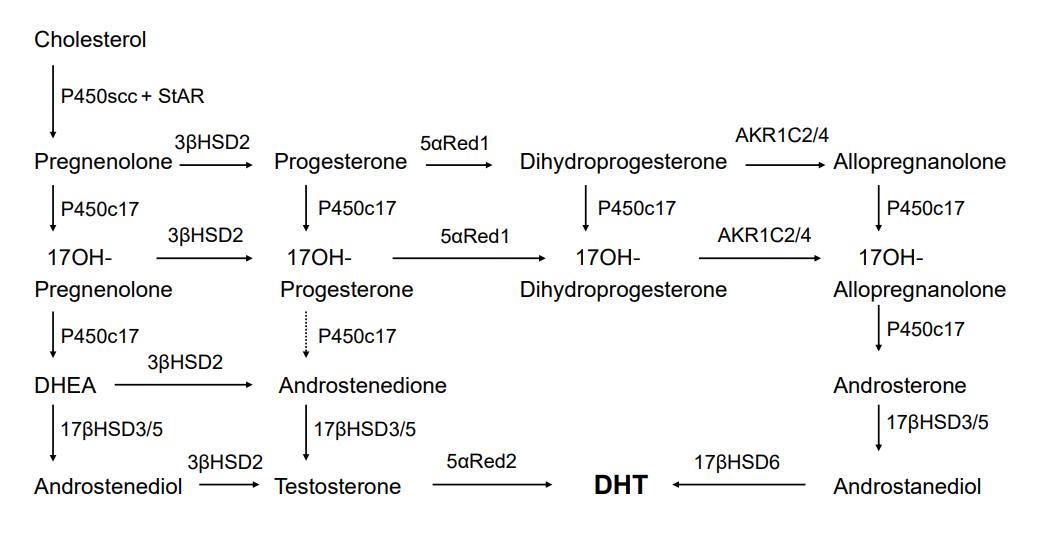
- Both genes and hormones regulate human sexual development. Although ovarian hormones are not essential for female external genitalia development, male sexual development requires the action of testicular testosterone and dihydrotestosterone (DHT). DHT is the most active endogenous androgen formed by the conversion of testosterone in genital skin. This synthesis route from cholesterol to DHT is called the conventional classic pathway. Recent investigations have reported an alternative ("backdoor") route for DHT formation that bypasses fetal testicular testosterone. This alternative route plays a crucial role in human hyperandrogenic disorders like congenital adrenal hyperplasia caused by P450c21 deficiency, polycystic ovary syndrome, and P450 oxidoreductase deficiency. In addition, mutations in AKR1C2 and AKR1C4, genes encoding 3α-reductases, have been implicated in disorders of sexual development, indicating that both the classic and backdoor routes are required for normal human male sexual development. More recently, androsterone was found to be the primary androgen of the human backdoor route. Androsterone and steroidal substrates specific to the backdoor route are predominantly found in the placenta, liver, and adrenal glands rather than in the testes. These findings are essential to understanding human sexual development.
Keyword
Figure
Cited by 1 articles
-
Overview of endocrine tumor syndromes manifesting as adrenal tumors
Ja Hye Kim
Ewha Med J. 2024;47(1):e4. doi: 10.12771/emj.2024.e4.
Reference
-
References
1. Witchel SF, Lee PA. Ambiguous genitalia. In : Sperling MA, editor. Pediatric endocrinology. 5th ed. Philadelphia (PA): Elsevier;2020. p. 123–74.2. Witchel SF. Disorders of sex development. Best Pract Res Clin Obstet Gynaecol. 2018; 48:90–102.3. Siiteri PK, Wilson JD. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab. 1974; 38:113–25.4. Wilson JD, Griffin JE, Russell DW. Steroid 5α-reductase 2 deficiency. Endocr Rev. 1993; 14:577–93.5. Miller WL, Auchus RJ. The "backdoor pathway" of androgen synthesis in human male sexual development. PLoS Biol. 2019; 17:e3000198.6. Fluck CE, Meyer-Boni M, Pandey AV, Kempna P, Miller WL, Schoenle EJ, et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011; 89:201–18.7. O'Shaughnessy PJ, Antignac JP, Le Bizec B, Morvan M-L, Svechnikov K, Soder O, et al. Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. 2019; 17:e3000002.8. Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative "backdoor" pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012; 97:E367–75.9. Fukami M, Homma K, Hasegawa T, Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Dev Dyn. 2013; 242:320–9.10. Miller WL, Auchus RJ. The molecular biology, biochemistry and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011; 32:81–151.11. Miller WL. Congenital lipoid adrenal hyperplasia: the human gene knockout for the steroidogenic acute regulatory protein. J Mol Endocrinol. 1997; 19:227–40.12. Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996; 17:221–44.13. Black SM, Harikrishna JA, Szklarz GD, Miller WL. The mitochondrial environment is required for activity of the cholesterol side-chain cleavage enzyme, cytochrome P450scc. Proc Natl Acad Sci U S A. 1994; 91:7247–51.14. Miller WL. Minireview: regulation of steroidogenesis by electron transfer. Endocrinology. 2005; 146:2544–50.15. Miller WL. Disorders of androgen synthesis-from cholesterol to dehydroepiandrosterone. Med Princ Pract. 2005; 14 Suppl 1:58–68.16. Renfree MB, Wilson D, Short RV, Shaw G, George FW. Steroid hormone content of the gonads of the tammar wallaby during sexual differentiation. Biol Reprod. 1992; 47:644–7.17. Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, et al. 5α-androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology. 2003; 144:575–80.18. Mahendroo M, Wilson JD, Richardson JA, Auchus RJ. Steroid 5α-reductase 1 promotes 5α-androstane-3α,17β-diol synthesis in immature mouse testes by two pathways. Mol Cell Endocrinol. 2004; 222:113–20.19. Butler CM, Shaw G, Renfree MB. Development of the penis and clitoris in the tammar wallaby, Macropus eugenii. Anat Embryol. 1999; 199:451–7.20. Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004; 15:432–8.21. Ghayee HK, Auchus RJ. Clinical implications of androgen synthesis via 5α-reduced precursors. Endocr Dev. 2008; 13:55–66.22. Frederiksen DW, Wilson JD. Partial characterization of the nuclear reduced nicotinamide adenine dinucleotide phosphate: Δ4-3-ketosteroid 5α-oxidoreductase of rat prostate. J Biol Chem. 1971; 246:2584–93.23. Gupta MK, Guryev OL, Auchus RJ. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003; 418:151–60.24. Marti N, Galvan JA, Pandey AV, Trippel M, Tapia C, Muller M, et al. Genes and proteins of the alternative steroid backdoor pathway for dihydrotestosterone synthesis are expressed in the human ovary and seem enhanced in the polycystic ovary syndrome. Mol Cell Endocrinol. 2017; 441:116–23.25. Shackleton C, Marcos J, Malunowicz EM, Szarras-Czapnik M, Jira P, Taylor NF, et al. Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am J Med Genet A. 2004; 128A:223–31.26. Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, et al. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab. 2006; 91:2643–9.27. Krone N, Reisch N, Idkowiak J, Dhir V, Ivison HE, Hughes BA, et al. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2012; 97:E257–67.28. Dhayat NA, Dick B, Frey BM, d’Uscio CH, Vogt B, Fluck CE. Androgen biosynthesis during minipuberty favors the backdoor pathway over the classic pathway: insights into enzyme activities and steroid fluxes in healthy infants during the first year of life from the urinary steroid metabolome. J Steroid Biochem Mol Biol. 2017; 165:312–22.29. Biason-Lauber A, Miller WL, Pandey AV, Fluck CE. Of marsupials and men: "Backdoor" dihydrotestosterone synthesis in male sexual differentiation. Mol Cell Endocrinol. 2013; 371:124–32.30. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000; 21:245–91.31. Gidlof S, Falhammar H, Thilen A, von Dobeln U, Ritzen M, Wedell A, et al. One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2013; 1:35–42.32. White PC, New MI, Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA. 1984; 81:7505–9.33. Krone N, Dhir V, Ivison HE, Arlt W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf). 2007; 66:162–72.34. Storbeck KH, Schiffer L, Baranowski ES, Chortis V, Prete A, Barnard L, et al. Steroid metabolome analysis in disorders of adrenal steroid biosynthesis and metabolism. Endocr Rev. 2019; 40:1605–25.35. Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998; 273:3158–65.36. Lin-Su K, Nimkarn S, New MI. Congenital adrenal hyperplasia in adolescents: diagnosis and management. Ann N Y Acad Sci. 2008; 1135:95–8.37. Goto M, Piper Hanley K, Marcos J, Wood PJ, Wright S, Postle AD, et al. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest. 2006; 116:953–60.38. Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol. 2010; 121:496–504.39. Miller WL. P450 oxidoreductase deficiency: a new disorder of steroidogenesis with multiple clinical manifestations. Trends Endocrinol Metab. 2004; 15:311–5.40. Scott RR, Miller WL. Genetic and clinical features of P450 oxidoreductase deficiency. Horm Res. 2008; 69:266–75.41. Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004; 363:2128–35.42. Fukami M, Nishimura G, Homma K, Nagai T, Hanaki K, Uematsu A, et al. Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab. 2009; 94:1723–31.43. Shackleton C, Marcos J, Arlt W, Hauffa BP. Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype. Am J Med Genet A. 2004; 129A:105–12.44. Fukami M, Horikawa R, Nagai T, Tanaka T, Naiki Y, Sato N, et al. Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab. 2005; 90:414–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Commentary on "Classic and backdoor pathways of androgen biosynthesis in human sexual development"
- Effect of Menopause on the Expression of Androgen Receptors in Human Vagina
- 5alpha-Reductase
- The Clinical Effect of Androgen Replacement Therapy for Female Sexual Dysfunction
- Expression of androgen receptors in astrocytoma