Korean J Physiol Pharmacol.
2022 Jul;26(4):229-238. kjpp.2022.26.4.229.
Glycine induces enhancement of bactericidal activity of neutrophils
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Hallym University, Chuncheon 24252, Korea
- 2Department of Physiology, School of Medicine, Kyungpook National University, Daegu 41944, Korea
- KMID: 2530941
- DOI: http://doi.org/kjpp.2022.26.4.229
Abstract
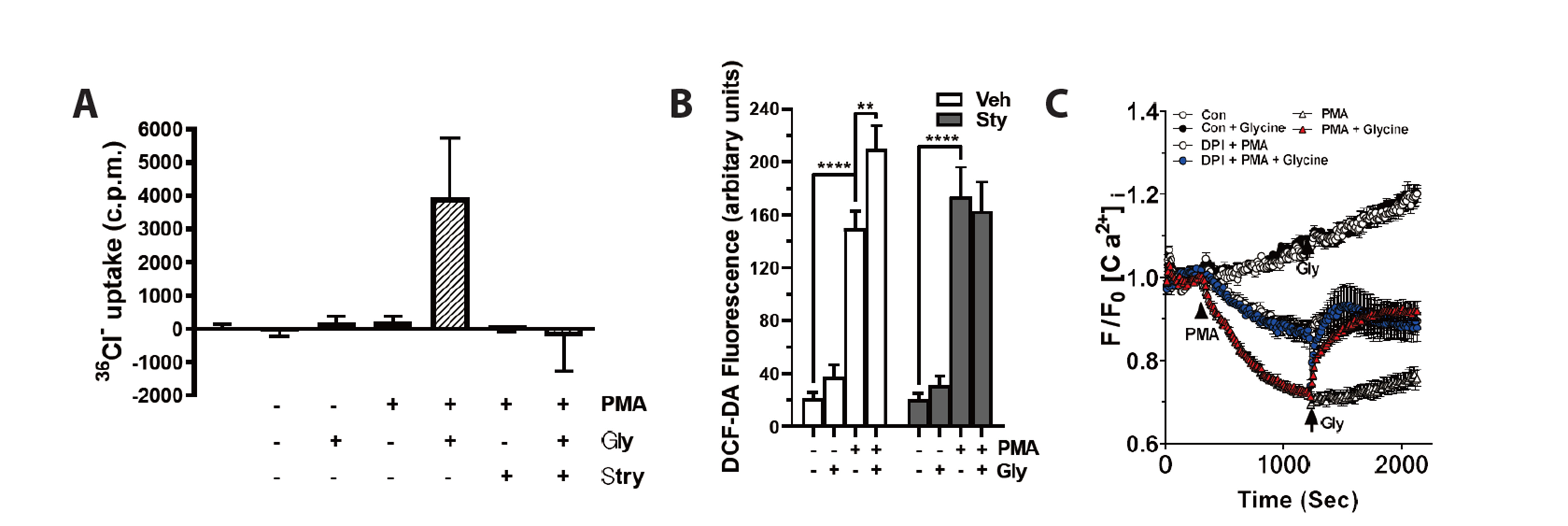
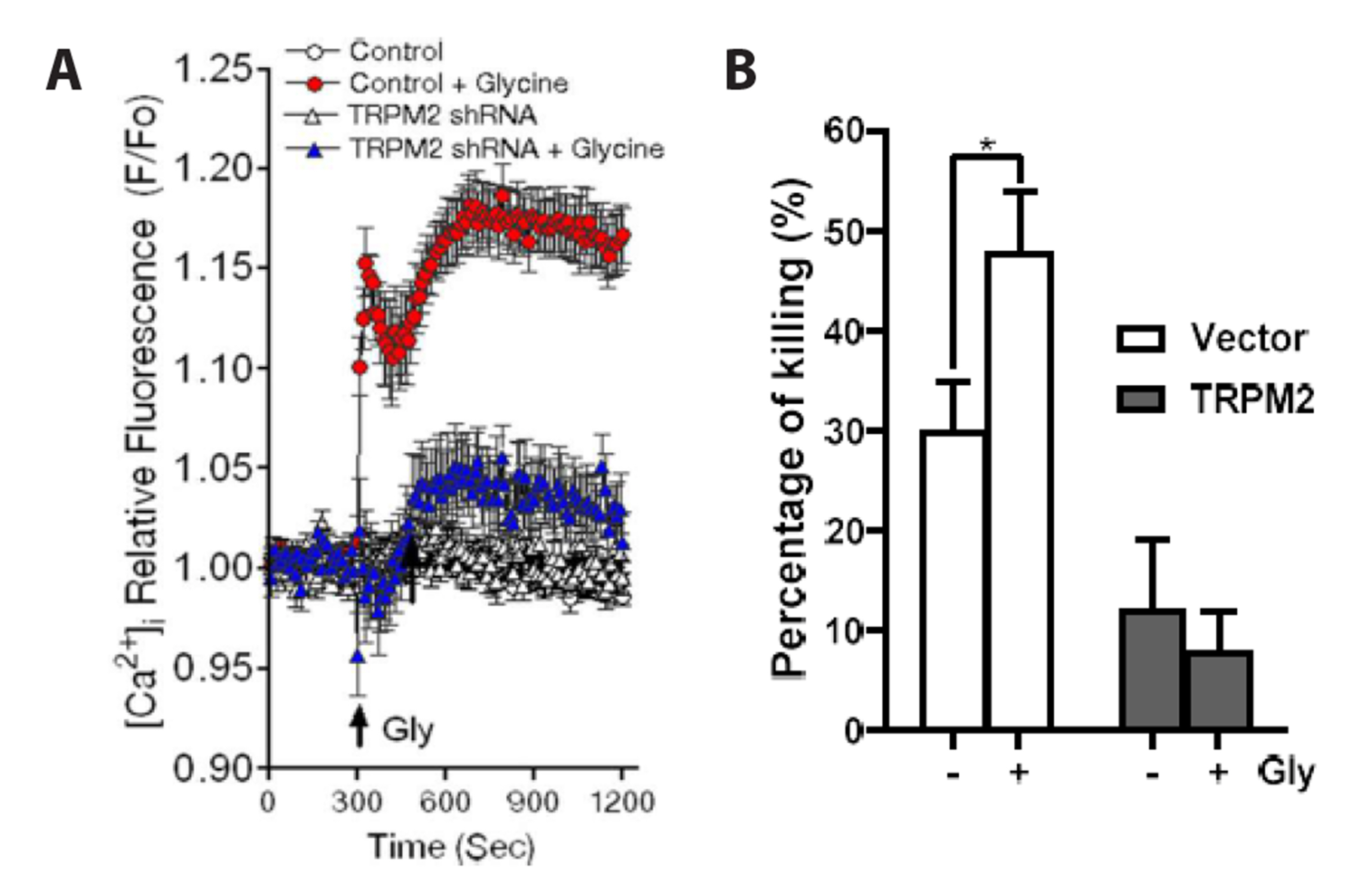
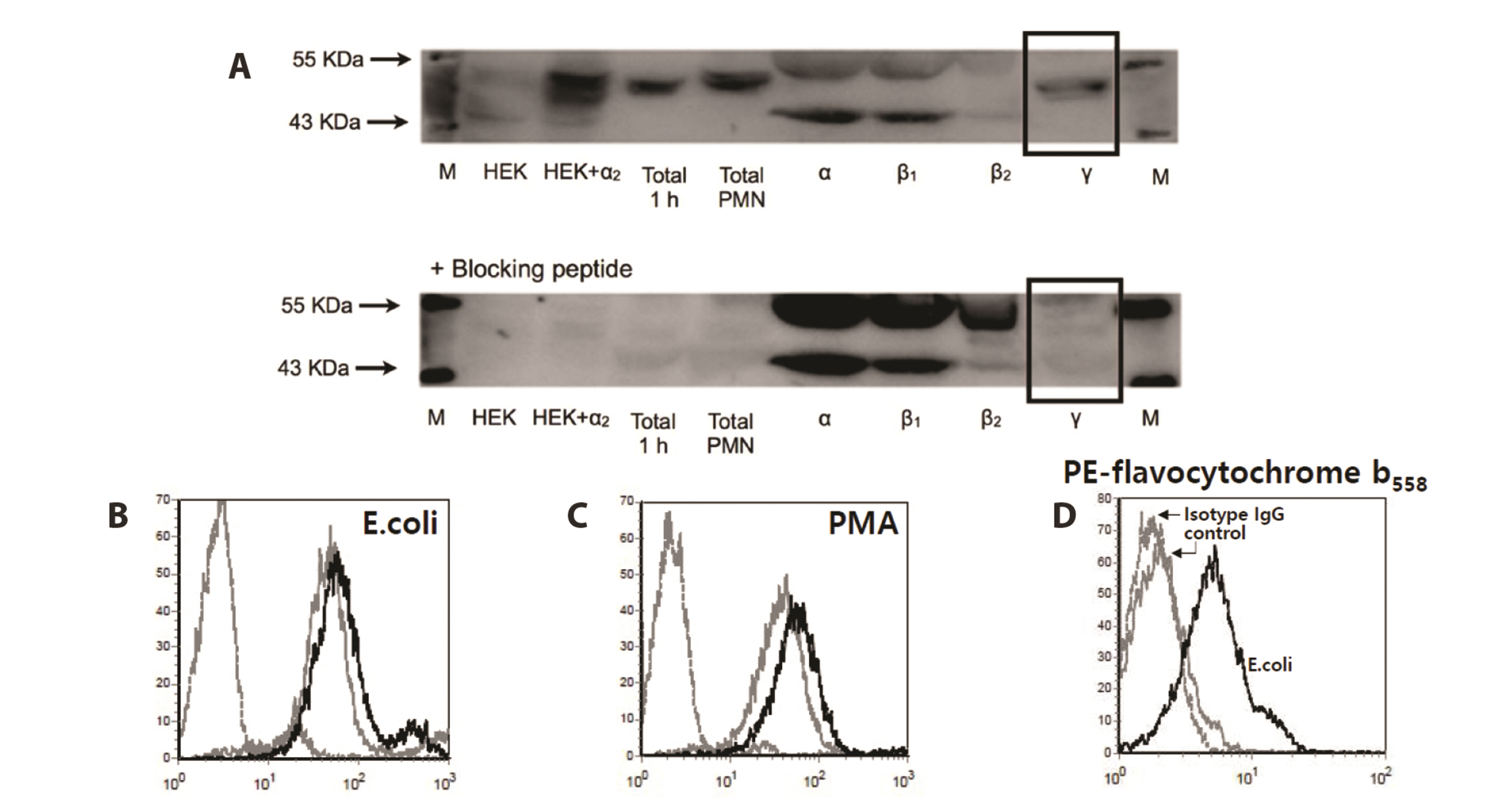
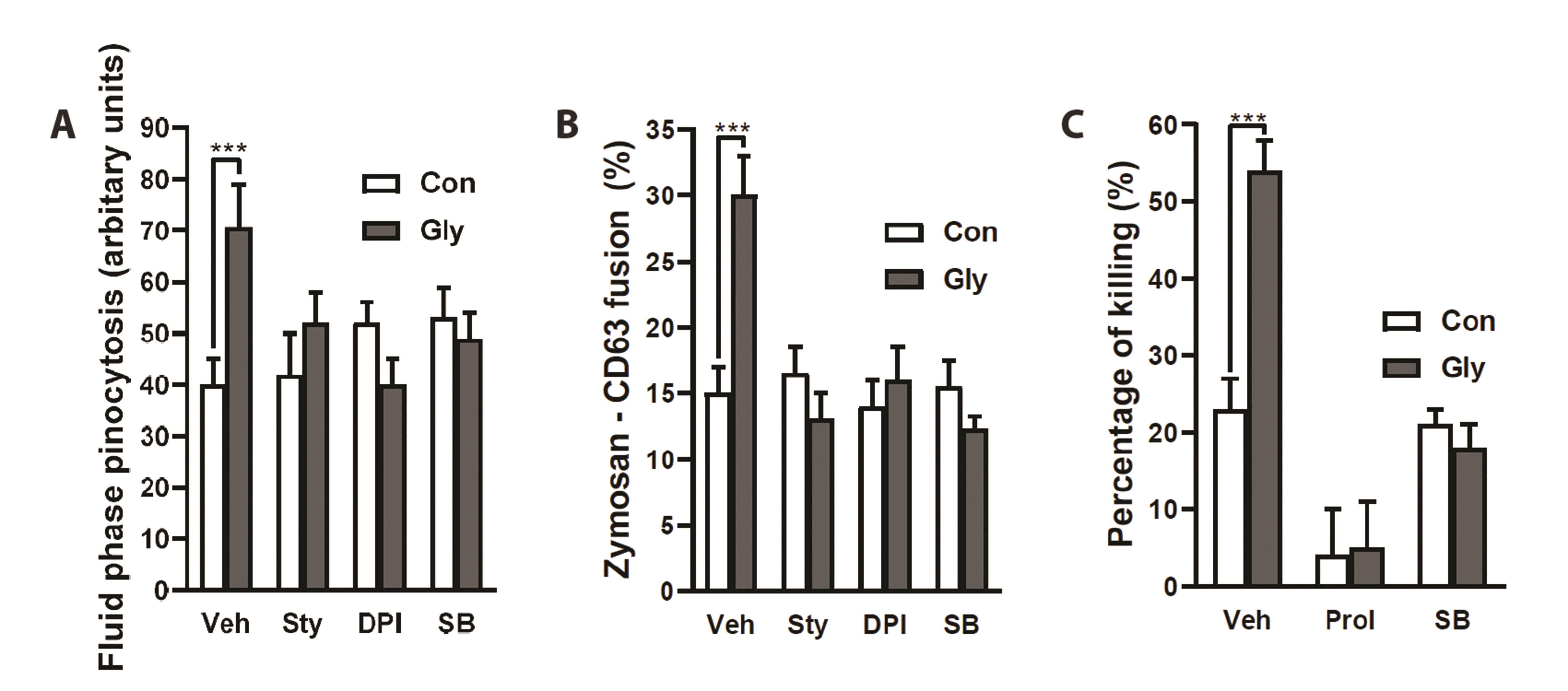
- Severe bacterial infections are frequently accompanied by depressed neutrophil functions. Thus, agents that increase the microbicidal activity of neutrophils could add to a direct antimicrobial therapy. Lysophosphatidylcholine augments neutrophil bactericidal activity via the glycine (Gly)/glycine receptor (GlyR) α2/ TRPM2/p38 mitogen-activated protein kinase (MAPK) pathway. However, the direct effect of glycine on neutrophil bactericidal activity was not reported. In this study, the effect of glycine on neutrophil bactericidal activity was examined. Glycine augmented bactericidal activity of human neutrophils (EC50 = 238 μM) in a strychnine (a GlyR antagonist)-sensitive manner. Glycine augmented bacterial clearance in mice, which was also blocked by strychnine (0.4 mg/kg, s.c.). Glycine enhanced NADPH oxidase-mediated reactive oxygen species (ROS) production and TRPM2-mediated [Ca2+ ]i increase in neutrophils that had taken up E. coli. Glycine augmented Lucifer yellow uptake (fluid-phase pinocytosis) and azurophil granule-phagosome fusion in neutrophils that had taken up E. coli in an SB203580 (a p38 MAPK inhibitor)-sensitive manner. These findings indicate that glycine augments neutrophil microbicidal activity by enhancing azurophil granule-phagosome fusion via the GlyRα2/ROS/calcium/ p38 MAPK pathway. We suggest that glycine could be a useful agent for increasing neutrophil bacterial clearance.
Keyword
Figure
Reference
-
1. Segal AW. 2005; How neutrophils kill microbes. Annu Rev Immunol. 23:197–223. DOI: 10.1146/annurev.immunol.23.021704.115653. PMID: 15771570. PMCID: PMC2092448. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=17644377258&origin=inward.
Article2. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. 2021; The Neutrophil. Immunity. 54:1377–1391. DOI: 10.1016/j.immuni.2021.06.006. PMID: 34260886. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85111770558&origin=inward.
Article3. Alexander JW, Meakins JL. 1972; A physiological basis for the development of opportunistic infections in man. Ann Surg. 176:273–287. DOI: 10.1097/00000658-197209000-00003. PMID: 4561323. PMCID: PMC1355390. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0015402530&origin=inward.
Article4. Solberg CO, Hellum KB. 1972; Neutrophil granulocyte function in bacterial infections. Lancet. 2:727–730. DOI: 10.1016/S0140-6736(72)92022-3. PMID: 4116144. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0015497270&origin=inward.
Article5. Christou NV, Meakins JL. 1979; Neutrophil function in anergic surgical patients: neutrophil adherence and chemotaxis. Ann Surg. 190:557–564. DOI: 10.1097/00000658-197911000-00001. PMID: 507967. PMCID: PMC1344532. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0018736120&origin=inward.6. Solomkin JS, Bauman MP, Nelson RD, Simmons RL. 1981; Neutrophils dysfunction during the course of intra-abdominal infection. Ann Surg. 194:9–17. DOI: 10.1097/00000658-198107000-00003. PMID: 7247540. PMCID: PMC1345188. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019792357&origin=inward.
Article7. Solomkin JS, Cotta LA, Brodt JK, Hurst JW, Ogle CK. 1984; Neutrophil dysfunction in sepsis. III. Degranulation as a mechanism for nonspecific deactivation. J Surg Res. 36:407–412. DOI: 10.1016/0022-4804(84)90119-7. PMID: 6328115. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0021276177&origin=inward.
Article8. Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. 1999; Protective effects of C5a blockade in sepsis. Nat Med. 5:788–792. DOI: 10.1038/10512. PMID: 10395324. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033052453&origin=inward.
Article9. Tavares-Murta BM, Zaparoli M, Ferreira RB, Silva-Vergara ML, Oliveira CH, Murta EF, Ferreira SH, Cunha FQ. 2002; Failure of neutrophil chemotactic function in septic patients. Crit Care Med. 30:1056–1061. DOI: 10.1097/00003246-200205000-00017. PMID: 12006803. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036256051&origin=inward.
Article10. Kaufmann I, Hoelzl A, Schliephake F, Hummel T, Chouker A, Peter K, Thiel M. 2006; Polymorphonuclear leukocyte dysfunction syndrome in patients with increasing sepsis severity. Shock. 26:254–261. DOI: 10.1097/01.shk.0000223131.64512.7a. PMID: 16912650. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33747330314&origin=inward.
Article11. Stephan F, Yang K, Tankovic J, Soussy CJ, Dhonneur G, Duvaldestin P, Brochard L, Brun-Buisson C, Harf A, Delclaux C. 2002; Impairment of polymorphonuclear neutrophil functions precedes nosocomial infections in critically ill patients. Crit Care Med. 30:315–322. DOI: 10.1097/00003246-200202000-00009. PMID: 11889301. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036172413&origin=inward.
Article12. Liles WC. 2001; Immunomodulatory approaches to augment phagocyte-mediated host defense for treatment of infectious diseases. Semin Respir Infect. 16:11–17. DOI: 10.1053/srin.2001.22724. PMID: 11309708. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035069871&origin=inward.
Article13. Hong CW, Kim TK, Ham HY, Nam JS, Kim YH, Zheng H, Pang B, Min TK, Jung JS, Lee SN, Cho HJ, Kim EJ, Hong IH, Kang TC, Lee J, Oh SB, Jung SJ, Kim SJ, Song DK. 2010; Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol. 184:4401–4413. DOI: 10.4049/jimmunol.0902814. PMID: 20237295. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77952751229&origin=inward.
Article14. Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. 2004; Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 10:161–167. DOI: 10.1038/nm989. PMID: 14716308. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=11144354207&origin=inward.
Article15. Blednov YA, Bleck V, Harris RA. 1996; Measurement of glycine receptor function by radioactive chloride uptake. J Neurosci Methods. 68:253–257. DOI: 10.1016/0165-0270(96)00088-X. PMID: 8912198. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030273551&origin=inward.
Article16. Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ. 2003; Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 102:1499–1505. DOI: 10.1182/blood-2002-12-3880. PMID: 12714492. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0043240071&origin=inward.
Article17. Fittschen C, Henson PM. 1994; Linkage of azurophil granule secretion in neutrophils to chloride ion transport and endosomal transcytosis. J Clin Invest. 93:247–255. DOI: 10.1172/JCI116952. PMID: 8282794. PMCID: PMC293759. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028123331&origin=inward.
Article18. Roos D, van Bruggen R, Meischl C. 2003; Oxidative killing of microbes by neutrophils. Microbes Infect. 5:1307–1315. DOI: 10.1016/j.micinf.2003.09.009. PMID: 14613774. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0242460542&origin=inward.
Article19. Winterbourn CC, Kettle AJ, Hampton MB. 2016; Reactive oxygen species and neutrophil function. Annu Rev Biochem. 85:765–792. DOI: 10.1146/annurev-biochem-060815-014442. PMID: 27050287. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84974721064&origin=inward.
Article20. Wilsson A, Lundqvist H, Gustafsson M, Stendahl O. 1996; Killing of phagocytosed Staphylococcus aureus by human neutrophils requires intracellular free calcium. J Leukoc Biol. 59:902–907. DOI: 10.1002/jlb.59.6.902. PMID: 8691076. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029974262&origin=inward.
Article21. Kjeldsen L, Sengelov H, Borregaard N. 1999; Subcellular fractionation of human neutrophils on Percoll density gradients. J Immunol Methods. 232:131–143. DOI: 10.1016/S0022-1759(99)00171-4. PMID: 10618515. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033386768&origin=inward.
Article22. Heiner I, Eisfeld J, Lückhoff A. 2003; Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium. 33:533–540. DOI: 10.1016/S0143-4160(03)00058-7. PMID: 12765698. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037974386&origin=inward.
Article23. Heiner I, Eisfeld J, Warnstedt M, Radukina N, Jüngling E, Lückhoff A. 2006; Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J. 398:225–232. DOI: 10.1042/BJ20060183. PMID: 16719842. PMCID: PMC1550310. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33748336294&origin=inward.
Article24. Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. 2002; LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 9:163–173. DOI: 10.1016/S1097-2765(01)00438-5. PMID: 11804595. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=18244391511&origin=inward.
Article25. Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A. 2002; Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 277:23150–23156. DOI: 10.1074/jbc.M112096200. PMID: 11960981. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037189484&origin=inward.
Article26. Klempner MS, Mikkelsen RB, Corfman DH, André-Schwartz J. 1980; Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 86:21–28. DOI: 10.1083/jcb.86.1.21. PMID: 7419575. PMCID: PMC2110662. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019312005&origin=inward.
Article27. Borregaard N, Heiple JM, Simons ER, Clark RA. 1983; Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 97:52–61. DOI: 10.1083/jcb.97.1.52. PMID: 6408102. PMCID: PMC2112494. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0020540731&origin=inward.
Article28. Moreland JG, Davis AP, Bailey G, Nauseef WM, Lamb FS. 2006; Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 281:12277–12288. DOI: 10.1074/jbc.M511030200. PMID: 16522634. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33744963881&origin=inward.
Article29. Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. 2005; Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 78:1025–1042. DOI: 10.1189/jlb.0804442. PMID: 16204621. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=27644514297&origin=inward.
Article30. Borregaard N, Sørensen OE, Theilgaard-Mönch K. 2007; Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 28:340–345. DOI: 10.1016/j.it.2007.06.002. PMID: 17627888. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34447561633&origin=inward.
Article31. Abramson JS, Lewis JC, Lyles DS, Heller KA, Mills EL, Bass DA. 1982; Inhibition of neutrophil lysosome-phagosome fusion associated with influenza virus infection in vitro. Role in depressed bactericidal activity. J Clin Invest. 69:1393–1397. DOI: 10.1172/JCI110580. PMID: 7085879. PMCID: PMC370213. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019968304&origin=inward.
Article32. Staali L, Bauer S, Mörgelin M, Björck L, Tapper H. 2006; Streptococcus pyogenes bacteria modulate membrane traffic in human neutrophils and selectively inhibit azurophilic granule fusion with phagosomes. Cell Microbiol. 8:690–703. DOI: 10.1111/j.1462-5822.2005.00662.x. PMID: 16548894. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33644870686&origin=inward.
Article33. Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. 1998; Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 4:615–618. DOI: 10.1038/nm0598-615. PMID: 9585238. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031836105&origin=inward.
Article34. Garcia R, Gusmani L, Murgia R, Guarnaccia C, Cinco M, Rottini G. 1998; Elastase is the only human neutrophil granule protein that alone is responsible for in vitro killing of Borrelia burgdorferi. Infect Immun. 66:1408–1412. DOI: 10.1128/IAI.66.4.1408-1412.1998. PMID: 9529060. PMCID: PMC108067. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032038653&origin=inward.
Article35. Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. 2000; Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 12:201–210. DOI: 10.1016/S1074-7613(00)80173-9. PMID: 10714686. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034144636&origin=inward.
Article36. Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. 2002; Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 416:291–297. DOI: 10.1038/416291a. PMID: 11907569. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037149510&origin=inward.
Article37. Blander JM, Medzhitov R. 2004; Regulation of phagosome maturation by signals from toll-like receptors. Science. 304:1014–1018. DOI: 10.1126/science.1096158. PMID: 15143282. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2342525996&origin=inward.
Article38. Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. 2001; The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 7:421–432. DOI: 10.1016/S1097-2765(01)00189-7. PMID: 11239470. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035105295&origin=inward.
Article39. Ward RA, Nakamura M, McLeish KR. 2000; Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J Biol Chem. 275:36713–36719. DOI: 10.1074/jbc.M003017200. PMID: 10976103. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034711296&origin=inward.
Article40. Schnyder B, Meunier PC, Car BD. 1998; Inhibition of kinases impairs neutrophil activation and killing of Staphylococcus aureus. Biochem J. 331(Pt 2):489–495. DOI: 10.1042/bj3310489. PMID: 9531489. PMCID: PMC1219380. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032522553&origin=inward.
Article41. Zhong B, Jiang K, Gilvary DL, Epling-Burnette PK, Ritchey C, Liu J, Jackson RJ, Hong-Geller E, Wei S. 2003; Human neutrophils utilize a Rac/Cdc42-dependent MAPK pathway to direct intracellular granule mobilization toward ingested microbial pathogens. Blood. 101:3240–3248. DOI: 10.1182/blood-2001-12-0180. PMID: 12511425. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0038281350&origin=inward.
Article42. Murphy R, DeCoursey TE. 2006; Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 1757:996–1011. DOI: 10.1016/j.bbabio.2006.01.005. PMID: 16483534. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33748894518&origin=inward.
Article43. Gold SB, Hanes DM, Stites DP, Fudenberg HH. 1974; Abnormal kinetics of degranulation in chronic granulomatous disease. N Engl J Med. 291:332–337. DOI: 10.1056/NEJM197408152910704. PMID: 4850362. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0016269090&origin=inward.
Article44. Ellis JA, Mayer SJ, Jones OT. 1988; The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem J. 251:887–891. DOI: 10.1042/bj2510887. PMID: 2843166. PMCID: PMC1149085. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0023894234&origin=inward.
Article45. Segal AW, Geisow M, Garcia R, Harper A, Miller R. 1981; The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 290:406–409. DOI: 10.1038/290406a0. PMID: 7219526. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0019480640&origin=inward.
Article46. Weiss J, Kao L, Victor M, Elsbach P. 1987; Respiratory burst facilitates the digestion of Escherichia coli killed by polymorphonuclear leukocytes. Infect Immun. 55:2142–2147. DOI: 10.1128/iai.55.9.2142-2147.1987. PMID: 3305366. PMCID: PMC260670.
Article47. Nauseef WM. 2007; How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 219:88–102. DOI: 10.1111/j.1600-065X.2007.00550.x. PMID: 17850484. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34548588559&origin=inward.
Article48. Baran H, Gramer M, Löscher W. 1995; Alterations in plasma and brain amino acids after administration of the glycine/NMDA receptor partial agonist, D-cycloserine, to mice and rats. Eur J Pharmacol. 273:197–201. DOI: 10.1016/0014-2999(94)00745-S. PMID: 7737315. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028897366&origin=inward.
Article49. Askanazi J, Elwyn DH, Kinney JM, Gump FE, Michelsen CB, Stinchfield FE, Fürst P, Vinnars E, Bergström J. 1978; Muscle and plasma amino acids after injury: the role of inactivity. Ann Surg. 188:797–803. DOI: 10.1097/00000658-197812000-00014. PMID: 736657. PMCID: PMC1397017. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0018106149&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Bactericidal Activity of Serum of Leprosy Patients
- Lysophosphatidylcholine induces azurophil granule translocation via Rho/Rho kinase/F-actin polymerization in human neutrophils
- Autophagy in neutrophils
- Evaluation of In Vitro Bactericidal Activity of Disinfectants against Major Nosocomial Pathogens
- Effect of Strychinine, a Glycine Inhibitor, on the Programmed Cell Death of Motoneurons during the Chick Development