J Stroke.
2022 May;24(2):266-277. 10.5853/jos.2021.01823.
Etiology, 3-Month Functional Outcome and Recurrent Events in Non-Traumatic Intracerebral Hemorrhage
- Affiliations
-
- 1Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 2Graduate School of Health Sciences, University of Bern, Bern, Switzerland
- 3Department of Neurology, University Hospital Zurich, University of Zurich, Zurich, Switzerland
- 4Service of Neurology, Department of Clinical Neurosciences, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland
- 5Department of Neurology, Buergerspital Solothurn, Solothurn, Switzerland
- 6Stroke Center EOC, Neurocenter of Southern Switzerland, Lugano, Switzerland
- 7Stroke Unit, Department of Internal Medicine, Hospital of Grabs, Grabs, Switzerland
- 8Stroke Unit and Division of Neurology, Department of Internal Medicine, HFR Fribourg–Cantonal Hospital, Villars-sur-Glâne, Switzerland
- 9Stroke Center Hirslanden, Klinik Hirslanden Zurich, Zurich, Switzerland
- 10Division of Neurology, Pourtalès Hospital, Neuchatel, Switzerland
- 11Stroke Unit, GHOL, Hospital Nyon, Nyon, Switzerland
- 12Stroke Research Group, Department of Clinical Neurosciences, Geneva University Hospital, Faculty of Medicine, University of Geneva, Geneva, Switzerland
- 13Service of Neurology, Valais Hospital, Sion, Switzerland
- 14Department of Internal Medicine, Hospital Graubünden, Chur, Switzerland
- 15Department of Neurology, Cantonal Hospital Aarau, Aarau, Switzerland
- 16Department of Neurology, Cantonal Hospital, St. Gallen, Switzerland
- 17Stroke Unit, Department of Neurology, Cantonal Hospital Winterthur (KSW), Winterthur, Switzerland
- 18Neurology Department, Lucerne Cantonal Hospital (LUKS), Luzern, Switzerland
- 19Division of Neurology, Kantonsspital Münsterlingen, Munsterlingen, Switzerland
- 20Stroke Unit, Department of Neurology, Hospital Biel, Biel, Switzerland
- 21Stadtspitäler Triemli und Waid, Zurich, Switzerland
- 22Department of Neurology and Stroke Center, University Hospital Basel, University of Basel, Basel, Switzerland
- 23Neurology and Neurorehabilitation, University Department of Geriatric Medicine FELIX PLATTER, University of Basel, Basel, Switzerland
- 24Cereneo Center for Neurology and Rehabilitation, Vitznau, Switzerland
- 25Department of Neurosurgery, Inselspital, Bern University Hospital, Bern, Switzerland
- 26Department of Neurology, University Hospital Erlangen, Friedrich-Alexander-University Erlangen-Nuremberg (FAU), Erlangen, Germany
- 27University Institute of Diagnostic and Interventional Neuroradiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 28University Institute of Diagnostic, Interventional and Paediatric Radiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 29Department of Neurosurgery, Cantonal Hospital Aarau, Aarau, Switzerland
- KMID: 2530529
- DOI: http://doi.org/10.5853/jos.2021.01823
Abstract
- Background and Purpose
Knowledge about different etiologies of non-traumatic intracerebral hemorrhage (ICH) and their outcomes is scarce.
Methods
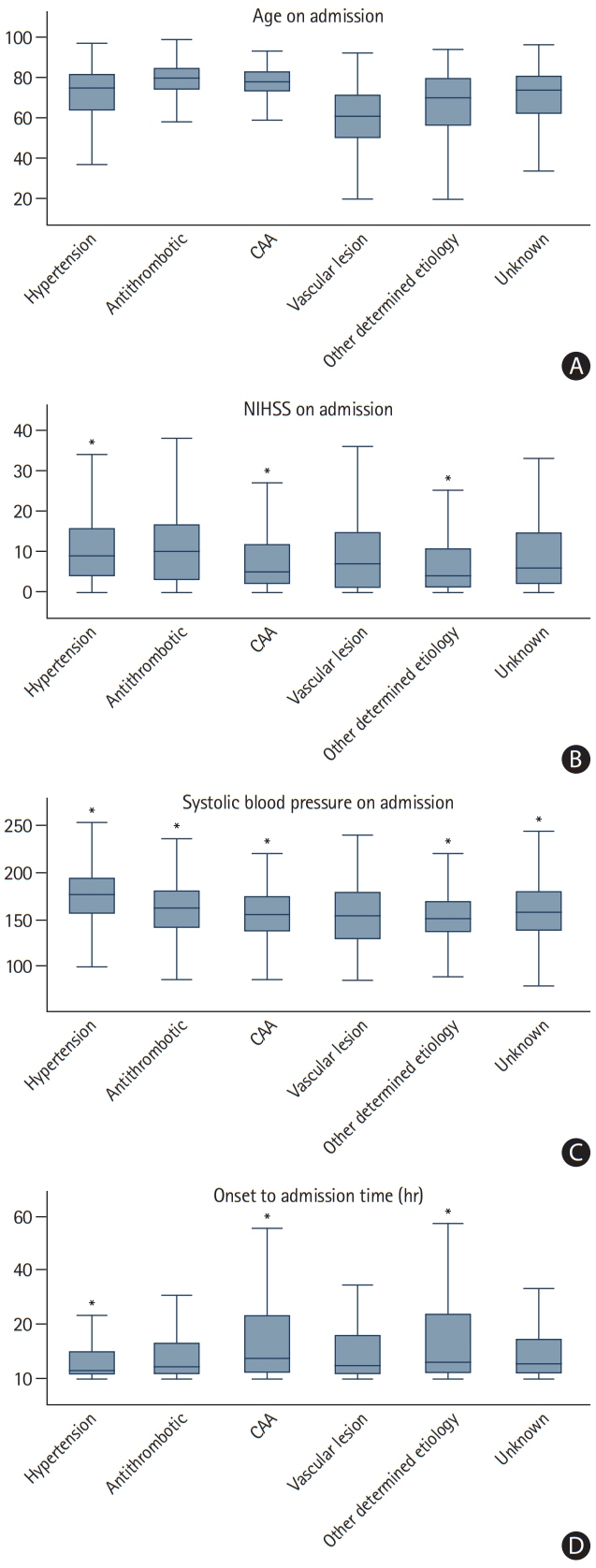
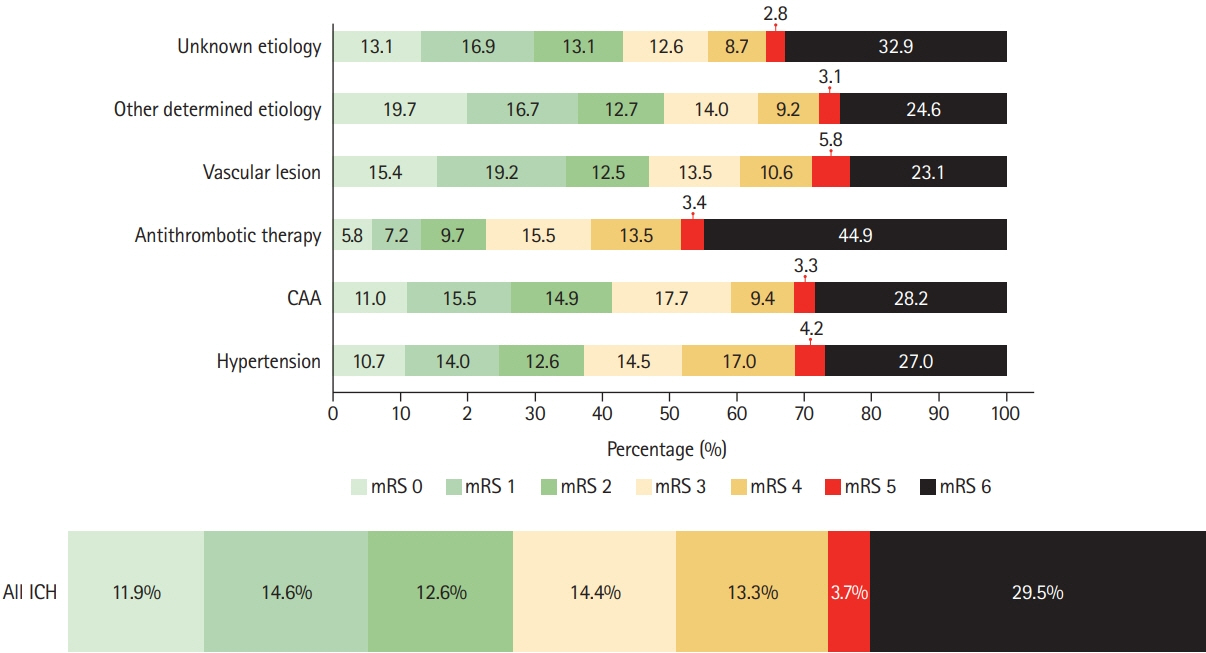
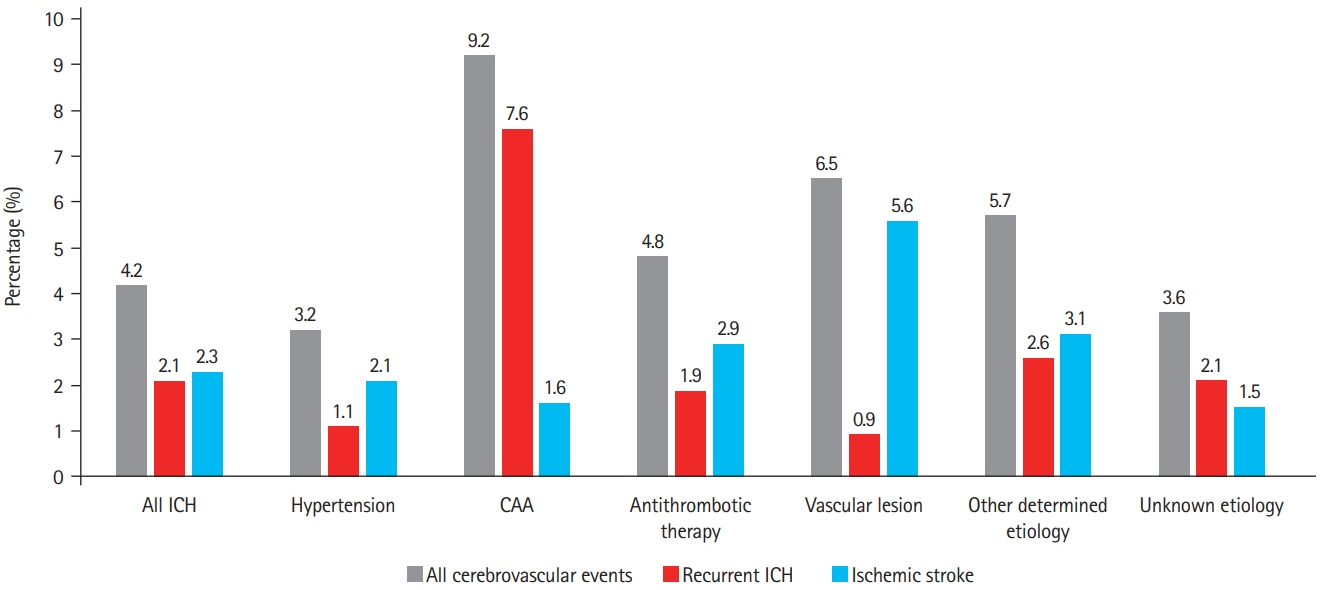
We assessed prevalence of pre-specified ICH etiologies and their association with outcomes in consecutive ICH patients enrolled in the prospective Swiss Stroke Registry (2014 to 2019). Results We included 2,650 patients (mean±standard deviation age 72±14 years, 46.5% female, median National Institutes of Health Stroke Scale 8 [interquartile range, 3 to 15]). Etiology was as follows: hypertension, 1,238 (46.7%); unknown, 566 (21.4%); antithrombotic therapy, 227 (8.6%); cerebral amyloid angiopathy (CAA), 217 (8.2%); macrovascular cause, 128 (4.8%); other determined etiology, 274 patients (10.3%). At 3 months, 880 patients (33.2%) were functionally independent and 664 had died (25.1%). ICH due to hypertension had a higher odds of functional independence (adjusted odds ratio [aOR], 1.33; 95% confidence interval [CI], 1.00 to 1.77; P=0.05) and lower mortality (aOR, 0.64; 95% CI, 0.47 to 0.86; P=0.003). ICH due to antithrombotic therapy had higher mortality (aOR, 1.62; 95% CI, 1.01 to 2.61; P=0.045). Within 3 months, 4.2% of patients had cerebrovascular events. The rate of ischemic stroke was higher than that of recurrent ICH in all etiologies but CAA and unknown etiology. CAA had high odds of recurrent ICH (aOR, 3.38; 95% CI, 1.48 to 7.69; P=0.004) while the odds was lower in ICH due to hypertension (aOR, 0.42; 95% CI, 0.19 to 0.93; P=0.031).
Conclusions
Although hypertension is the leading etiology of ICH, other etiologies are frequent. One-third of ICH patients are functionally independent at 3 months. Except for patients with presumed CAA, the risk of ischemic stroke within 3 months of ICH was higher than the risk of recurrent hemorrhage.
Keyword
Figure
Reference
-
References
1. Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018; 392:1257–1268.
Article2. Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother. 2019; 19:679–694.
Article3. Shoamanesh A, Patrice Lindsay M, Castellucci LA, Cayley A, Crowther M, de Wit K, et al. Canadian stroke best practice recommendations: management of spontaneous intracerebral hemorrhage, 7th edition update 2020. Int J Stroke. 2021; 16:321–341.4. Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014; 9:840–855.
Article5. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
Article6. Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc Dis. 2009; 27:493–501.
Article7. Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007; 38:2979–2984.
Article8. Rannikmäe K, Woodfield R, Anderson CS, Charidimou A, Chiewvit P, Greenberg SM, et al. Reliability of intracerebral hemorrhage classification systems: a systematic review. Int J Stroke. 2016; 11:626–636.
Article9. Banerjee G, Wilson D, Ambler G, Hostettler IC, Shakeshaft C, Cohen H, et al. Longer term stroke risk in intracerebral haemorrhage survivors. J Neurol Neurosurg Psychiatry. 2020; 91:840–845.
Article10. Casolla B, Moulin S, Kyheng M, Hénon H, Labreuche J, Leys D, et al. Five-year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke. 2019; 50:1100–1107.
Article11. Charidimou A, Imaizumi T, Moulin S, Biffi A, Samarasekera N, Yakushiji Y, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta-analysis. Neurology. 2017; 89:820–829.
Article12. Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014; 85:660–667.
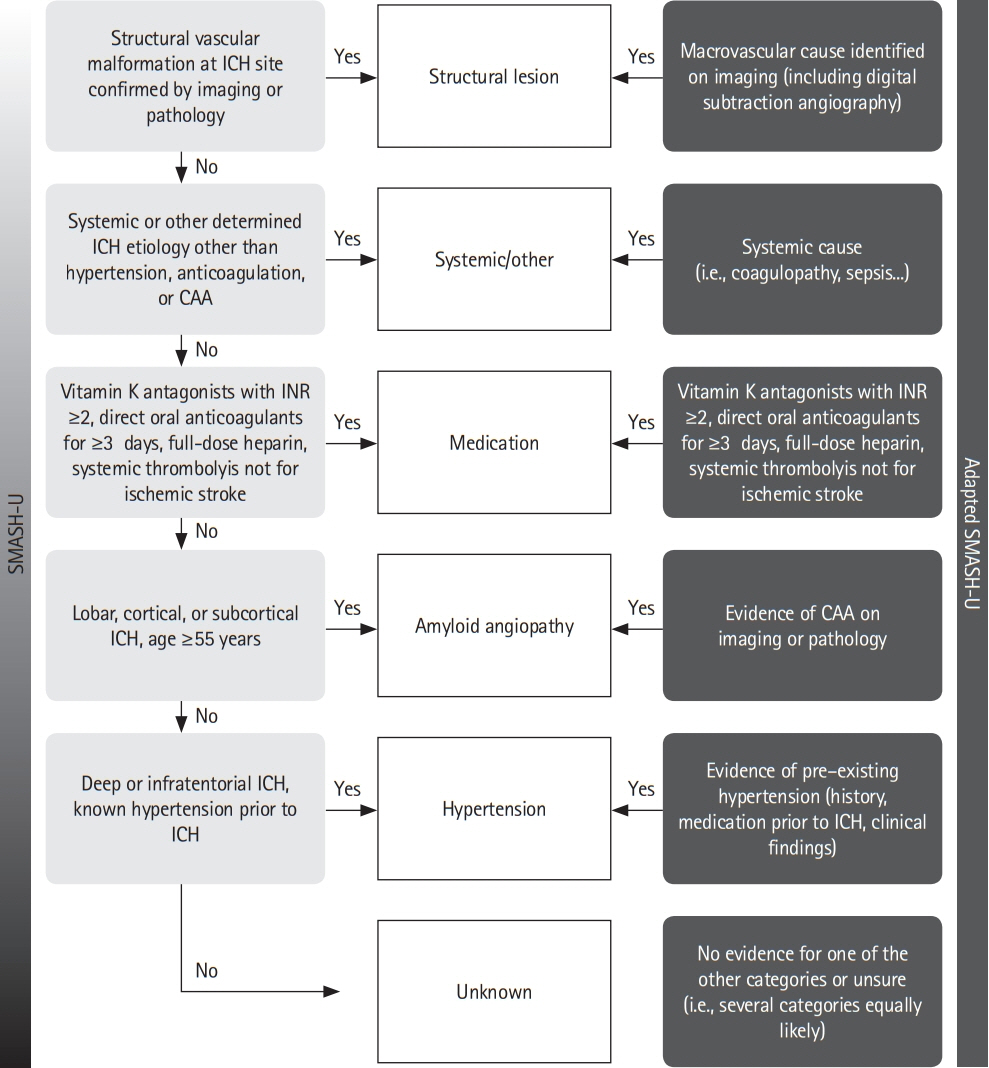
Article13. Meretoja A, Strbian D, Putaala J, Curtze S, Haapaniemi E, Mustanoja S, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012; 43:2592–2597.14. Martí-Fàbregas J, Prats-Sánchez L, Guisado-Alonso D, Martínez-Domeño A, Delgado-Mederos R, Camps-Renom P. SMASH-U versus H-ATOMIC: a head-to-head comparison for the etiologic classification of intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2018; 27:2375–2380.
Article15. Martí-Fàbregas J, Prats-Sánchez L, Martínez-Domeño A, Camps-Renom P, Marín R, Jiménez-Xarrié E, et al. The H-ATOMIC criteria for the etiologic classification of patients with intracerebral hemorrhage. PLoS One. 2016; 11:e0156992.
Article16. Ringelstein EB, Chamorro A, Kaste M, Langhorne P, Leys D, Lyrer P, et al. European Stroke Organisation recommendations to establish a stroke unit and stroke center. Stroke. 2013; 44:828–840.
Article17. Manno C, Disanto G, Bianco G, Nannoni S, Heldner M, Jung S, et al. Outcome of endovascular therapy in stroke with large vessel occlusion and mild symptoms. Neurology. 2019; 93:e1618–e1626.
Article18. Meinel TR, Branca M, De Marchis GM, Nedeltchev K, Kahles T, Bonati L, et al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol. 2021; 89:42–53.
Article19. Rodrigues MA, Samarasekera N, Lerpiniere C, Humphreys C, McCarron MO, White PM, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. 2018; 17:232–240.
Article20. Varmdal T, Ellekjær H, Fjærtoft H, Indredavik B, Lydersen S, Bonaa KH. Inter-rater reliability of a national acute stroke register. BMC Res Notes. 2015; 8:584.
Article21. Meretoja A, Yassi N, Wu TY, Churilov L, Sibolt G, Jeng JS, et al. Tranexamic acid in patients with intracerebral haemorrhage (STOP-AUST): a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2020; 19:980–987.
Article22. Béjot Y, Cordonnier C, Durier J, Aboa-Eboulé C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain. 2013; 136(Pt 2):658–664.
Article23. Seiffge DJ, Curtze S, Dequatre-Ponchelle N, Pezzini A, Tatlisumak T, Cordonnier C, et al. Hematoma location and morphology of anticoagulation-associated intracerebral hemorrhage. Neurology. 2019; 92:e782–e791.
Article24. Seiffge DJ, Wilson D, Ambler G, Banerjee G, Hostettler IC, Houlden H, et al. Small vessel disease burden and intracerebral haemorrhage in patients taking oral anticoagulants. J Neurol Neurosurg Psychiatry. 2021; 92:805–14.
Article25. Palm F, Henschke N, Wolf J, Zimmer K, Safer A, Schröder RJ, et al. Intracerebral haemorrhage in a population-based stroke registry (LuSSt): incidence, aetiology, functional outcome and mortality. J Neurol. 2013; 260:2541–2550.
Article26. Lioutas VA, Wu B, Norton C, Helenius J, Modak J, Selim M. Cerebral small vessel disease burden and functional and radiographic outcomes in intracerebral hemorrhage. J Neurol. 2018; 265:2803–2814.
Article27. Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018; 391:2107–2115.
Article28. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019; 393:1021–1032.29. Sembill JA, Gerner ST, Volbers B, Bobinger T, Lücking H, Kloska SP, et al. Severity assessment in maximally treated ICH patients: the max-ICH score. Neurology. 2017; 89:423–431.
Article30. Tilling EJ, El Tawil S, Muir KW. Do clinicians overestimate the severity of intracerebral hemorrhage? Stroke. 2019; 50:344–348.
Article31. Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015; 313:824–836.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Traumatic Carotid-cavernous Fistula Bringing about Intracerebral Hemorrhage
- Recurrent Hypertensive Intracerebral Hemorrhage
- Stereotactic Surgical Management of Traumatic Intracerebral Hematoma
- Etiology and Pathogenesis of Hypertensive Intracerebral Hemorrhage
- A Clinical Analysis of Delayed Traumatic Intracerebral Hemorrhage