Intest Res.
2022 Apr;20(2):260-268. 10.5217/ir.2021.00124.
One-year clinical efficacy and safety of indigo naturalis for active ulcerative colitis: a real-world prospective study
- Affiliations
-
- 1Department of Medicine and Clinical Science, Graduate School of Medical Sciences, Fukuoka, Japan
- 2Division of Genomics, Medical Institute of Bioregulation, Kyushu University, Fukuoka, Japan
- 3International Medical Department, Kyushu University Hospital, Fukuoka, Japan
- 4Department of Epidemiology and Public Health, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 5Department of Anatomic Pathology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 6Division of Gastroenterology, Department of Internal Medicine, Saga University, Saga, Japan
- KMID: 2529572
- DOI: http://doi.org/10.5217/ir.2021.00124
Abstract
- Background/Aims
Recent studies suggested a favorable effect of indigo naturalis (IN) in inducing remission for refractory ulcerative colitis (UC), however, the maintenance effect of IN for patients with UC remains unknown. Therefore, we conducted a prospective uncontrolled open-label study to analyze the efficacy and safety of IN for patients with UC.
Methods
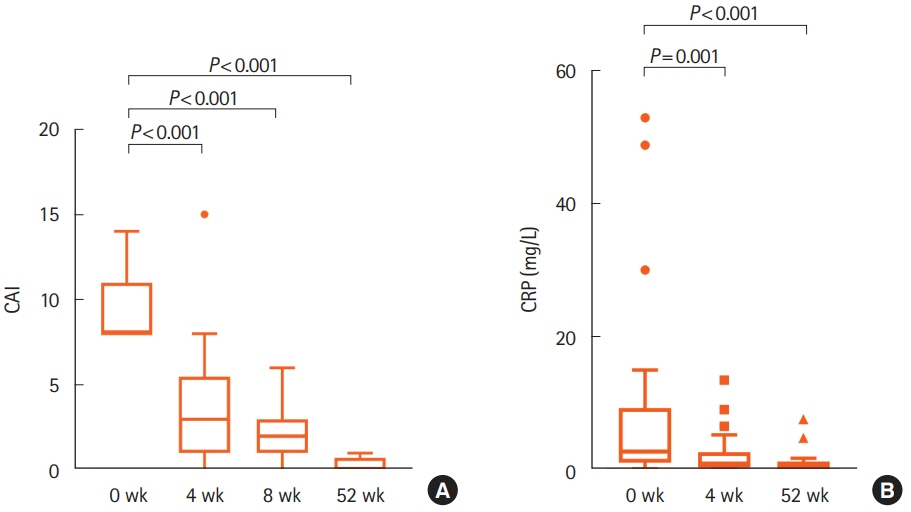
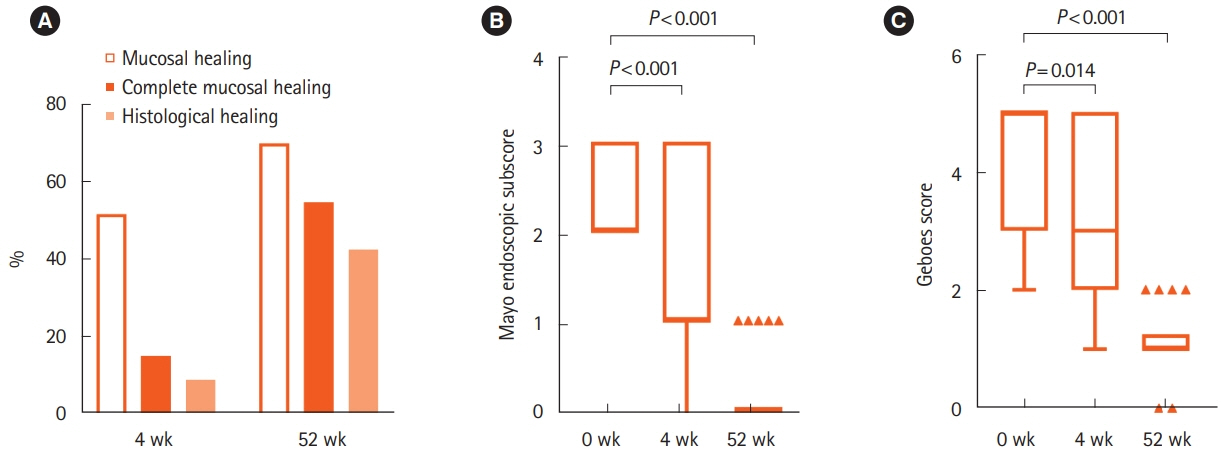
Patients with moderate to severe active UC (clinical activity index [CAI] ≥ 8) took 2 g/day of IN for 52 weeks. CAI at weeks 0, 4, 8, and 52 and Mayo endoscopic subscore (MES) and Geboes score (GS) at weeks 0, 4, and 52 were assessed. Clinical remission (CAI ≤ 4), mucosal healing (MES ≤ 1), and histological healing (GS ≤ 1) rates at each assessment were evaluated. Overall adverse events (AEs) during study period were also evaluated. The impact of IN on mucosal microbial composition was assessed using 16S ribosomal RNA gene sequences.
Results
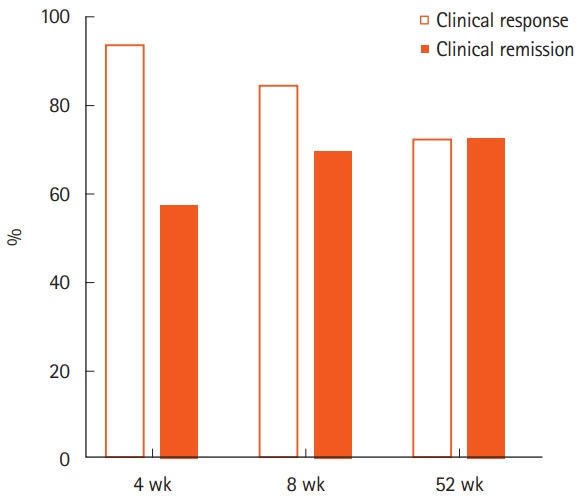
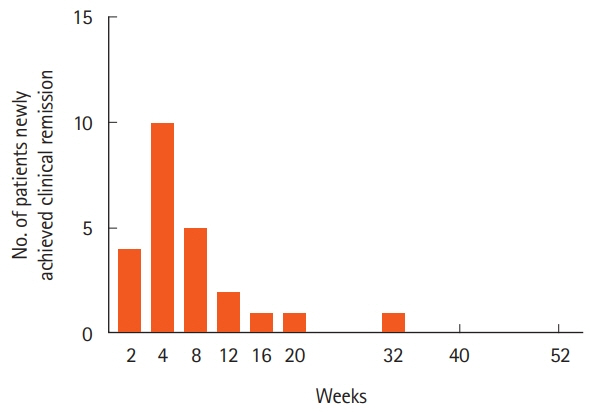
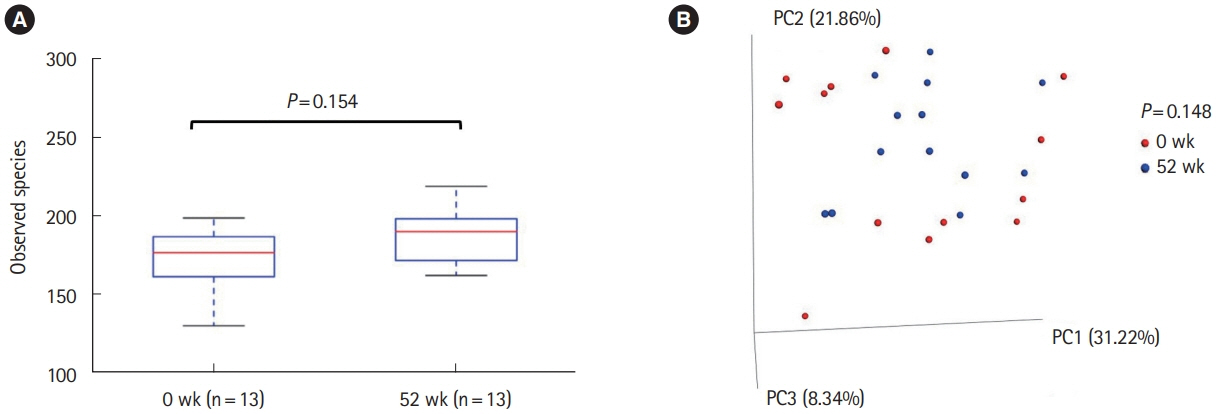
Thirty-three patients were enrolled. The rates of clinical remission at weeks 4, 8, and 52 were 67%, 76%, and 73%, respectively. The rates of mucosal healing at weeks 4 and 52 were 48% and 70%, respectively. AEs occurred in 17 patients (51.5%) during follow-up. Four patients (12.1%) showed severe AEs, among whom 3 manifested acute colitis. No significant alteration in the mucosal microbial composition was observed with IN treatment.
Conclusions
One-year treatment of moderate to severe UC with IN was effective. IN might be a promising therapeutic option for maintaining remission in UC, although the relatively high rate of AEs should be considered.
Figure
Reference
-
1. Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994; 107:3–11.
Article2. Hoie O, Wolters FL, Riis L, et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology. 2007; 132:507–515.
Article3. Adachi J, Mori Y, Matsui S, et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001; 276:31475–1478.
Article4. Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011; 141:237–248.
Article5. Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018; 53:465–474.
Article6. Kawai S, Iijima H, Shinzaki S, et al. Indigo naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol. 2017; 52:904–919.
Article7. Qiu J, Guo X, Chen ZM, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013; 39:386–399.
Article8. Behnsen J, Raffatellu M. Keeping the peace: aryl hydrocarbon receptor signaling modulates the mucosal microbiota. Immunity. 2013; 39:206–207.
Article9. Suzuki H, Kaneko T, Mizokami Y, et al. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J Gastroenterol. 2013; 19:2718–2722.
Article10. Naganuma M, Sugimoto S, Mitsuyama K, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018; 154:935–947.11. Matsuno Y, Hirano A, Torisu T, et al. Short-term and long-term outcomes of indigo naturalis treatment for inflammatory bowel disease. J Gastroenterol Hepatol. 2020; 35:412–417.
Article12. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article13. Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989; 298:82–86.
Article14. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article15. Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Guta. 2000; 47:404–409.
Article16. Nishio M, Hirooka K, Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur Heart J. 2016; 37:1992.
Article17. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.
Article18. Hirano A, Umeno J, Okamoto Y, et al. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J Gastroenterol Hepatol. 2018; 33:1590–1597.
Article19. Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012; 6:1621–1624.
Article20. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7:335–336.
Article21. Kondo S, Araki T, Okita Y, et al. Colitis with wall thickening and edematous changes during oral administration of the powdered form of Qing-Dai in patients with ulcerative colitis: a report of two cases. Clin J Gastroenterol. 2018; 11:268–272.
Article22. Yanai S, Nakamura S, Matsumoto T. Indigo naturalis-induced colitis. Dig Endosc. 2018; 30:791.
Article23. Matsuno Y, Hirano A, Esaki M. Possible association of phlebitisinduced colitis with indigo naturalis. Gastroenterology. 2018; 155:576–577.
Article24. Kim HA, Suh HR, Kang B, Choe BH; Crohn’s and Colitis Association in Daegu-Gyeongbuk (CCAiD). Acute pancreatitis associated with indigo naturalis in pediatric severe Crohn’s disease. Intest Res. 2019; 17:144–148.
Article25. Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019; 54:891–896.
Article26. Nishio M, Hirooka K, Doi Y. Pulmonary arterial hypertension associated with the Chinese herb indigo naturalis for ulcerative colitis: it may be reversible. Gastroenterology. 2018; 155:577–578.
Article27. Sato K, Ohira H, Horinouchi T, et al. Chinese herbal medicine Qing-Dai-induced pulmonary arterial hypertension in a patient with ulcerative colitis: a case report and experimental investigation. Respir Med Case Rep. 2019; 26:265–269.
Article28. Su C. Outcomes of placebo therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2006; 12:328–333.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute pancreatitis associated with indigo naturalis in pediatric severe Crohn’s disease
- Current new challenges in the management of ulcerative colitis
- Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study
- Usefulness of Magnifying Chromoscopy in Ulcerative Colitis
- Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: results from a large-scale, prospective, multicenter, observational study