Endocrinol Metab.
2022 Apr;37(2):233-242. 10.3803/EnM.2021.1353.
Comparative Study of Ex Vivo Antiplatelet Activity of Aspirin and Cilostazol in Patients with Diabetes and High Risk of Cardiovascular Disease
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2529215
- DOI: http://doi.org/10.3803/EnM.2021.1353
Abstract
- Background
The role of aspirin in primary cardiovascular disease prevention in patients with diabetes remains controversial. However, some studies have suggested beneficial effects of cilostazol on cardiovascular disease in patients with diabetes. We prospectively investigated the antiplatelet effects of cilostazol compared with aspirin in patients with diabetes and cardiovascular risk factors.
Methods
We randomly assigned 116 patients with type 2 diabetes and cardiovascular risk factors but no evident cardiovascular disease to receive aspirin at a dose of 100 mg or cilostazol at a dose of 200 mg daily for 14 days. The primary efficacy outcome was antiplatelet effects of aspirin and cilostazol assessed with the VerifyNow system (aspirin response units [ARU]) and PFA-100 (closure time [CT]). Secondary outcomes were changes of clinical laboratory data (ClinicalTrials.gov Identifier: NCT02933788).
Results
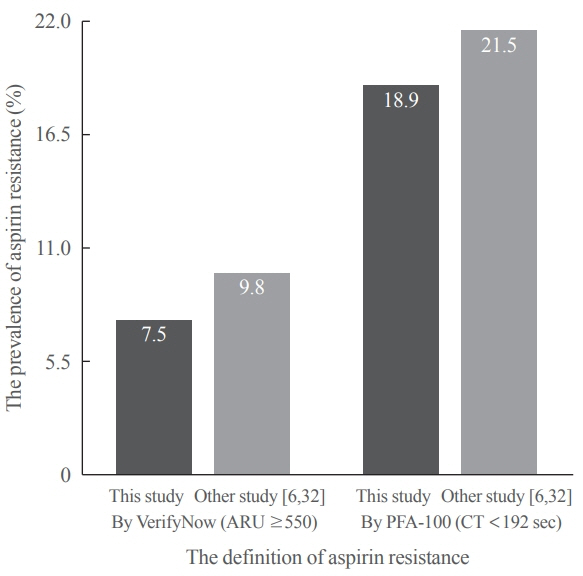
After 14 days, there was greater decrease in ARU in aspirin (–28.9%±9.9%) compared cilostazol (–0.4%±7.1%, P<0.001) and was greater increase in CT in aspirin (99.6%±63.5%) compared cilostazol (25.7%±54.1%, P<0.001). The prevalence of aspirin resistance was 7.5% according to VerifyNow (defined by ARU ≥550) and 18.9% according to PFA-100 (CT <192 seconds). Compared with aspirin, cilostazol treatment was associated with increased high density lipoprotein cholesterol (7.1%±12.7% vs. 4.2%±18.0%, P=0.006) and decreased triglycerides (–9.4%±33.7% vs. 4.4%±17.57%, P=0.016). However, there were no significant changes in total and low density lipoprotein cholesterol, C-reactive protein level, and cluster of differentiation 40 ligand between cilostazol and aspirin groups.
Conclusion
Aspirin showed better antiplatelet effects assessed with VerifyNow and PFA-100 compared with cilostazol. However, there were favorable changes in atherogenic dyslipidemia only in the cilostazol.
Keyword
Figure
Reference
-
1. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019; 43:398–406.
Article2. Antithrombotic Trialists’ Collaboration. Collaborative metaanalysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002; 324:71–86.3. Saito Y, Okada S, Ogawa H, Soejima H, Sakuma M, Nakayama M, et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-year follow-up of a randomized controlled trial. Circulation. 2017; 135:659–70.
Article4. ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018; 379:1529–39.
Article5. Al-Sofiani ME, Derenbecker R, Quartuccio M, Kalyani RR. Aspirin for primary prevention of cardiovascular disease in diabetes: a review of the evidence. Curr Diab Rep. 2019; 19:107.
Article6. Kim JD, Park CY, Ahn KJ, Cho JH, Choi KM, Kang JG, et al. Non-HDL cholesterol is an independent risk factor for aspirin resistance in obese patients with type 2 diabetes. Atherosclerosis. 2014; 234:146–51.
Article7. Kuliczkowski W, Witkowski A, Polonski L, Watala C, Filipiak K, Budaj A, et al. Interindividual variability in the response to oral antiplatelet drugs: a position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2009; 30:426–35.
Article8. Simpson SH, Abdelmoneim AS, Omran D, Featherstone TR. Prevalence of high on-treatment platelet reactivity in diabetic patients treated with aspirin. Am J Med. 2014; 127:95.
Article9. Uehara S, Hirayama A. Effects of cilostazol on platelet function. Arzneimittelforschung. 1989; 39:1531–4.10. Yeung J, Holinstat M. Newer agents in antiplatelet therapy: a review. J Blood Med. 2012; 3:33–42.11. Araki S, Matsuno H, Haneda M, Koya D, Kanno Y, Kume S, et al. Cilostazol attenuates spontaneous microaggregation of platelets in type 2 diabetic patients with insufficient platelet response to aspirin. Diabetes Care. 2013; 36:e92–3.
Article12. Shen H, Herzog W, Drolet M, Pakyz R, Newcomer S, Sack P, et al. Aspirin resistance in healthy drug-naive men versus women (from the Heredity and Phenotype Intervention Heart Study). Am J Cardiol. 2009; 104:606–12.
Article13. Schwartz KA. Aspirin resistance: a review of diagnostic methodology, mechanisms, and clinical utility. Adv Clin Chem. 2006; 42:81–110.
Article14. Muir AR, McMullin MF, Patterson C, McKeown PP. Assessment of aspirin resistance varies on a temporal basis in patients with ischaemic heart disease. Heart. 2009; 95:1225–9.
Article15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.
Article16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–70.
Article17. Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015; 11:133–48.
Article18. Kim JS, Lee KS, Kim YI, Tamai Y, Nakahata R, Takami H. A randomized crossover comparative study of aspirin, cilostazol and clopidogrel in normal controls: analysis with quantitative bleeding time and platelet aggregation test. J Clin Neurosci. 2004; 11:600–2.
Article19. Kariyazono H, Nakamura K, Arima J, Ayukawa O, Onimaru S, Masuda H, et al. Evaluation of anti-platelet aggregatory effects of aspirin, cilostazol and ramatroban on platelet-rich plasma and whole blood. Blood Coagul Fibrinolysis. 2004; 15:157–67.
Article20. Van Oosterom N, Barras M, Cottrell N, Bird R. Platelet function assays for the diagnosis of aspirin resistance. Platelets. 2022; 33:329–38.
Article21. Park JS, Kim YJ. The clinical effects of cilostazol on atherosclerotic vascular disease. Korean Circ J. 2008; 38:441–5.
Article22. Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007; 357:2482–94.
Article23. Ohta M, Satoh K, Fukasawa I, Hosokawa K, Oonishi T, Nakagomi J, et al. Assessment of cilostazol inhibition using whole blood samples: comparison of three platelet function tests. Yamanashi Med J. 2017; 32:27–37.24. Kim JY, Lee K, Shin M, Ahn M, Choe H, Yoo BS, et al. Cilostazol could ameliorate platelet responsiveness to clopidogrel in patients undergoing primary percutaneous coronary intervention. Circ J. 2007; 71:1867–72.
Article25. Lee JH, Cha JK, Lee SJ, Ha SW, Kwon SU. Addition of cilostazol reduces biological aspirin resistance in aspirin users with ischaemic stroke: a double-blind randomized clinical trial. Eur J Neurol. 2010; 17:434–42.
Article26. Kim CW, Yun JW, Bae IH, Park YH, Jeong YS, Park JW, et al. Evaluation of anti-platelet and anti-thrombotic effects of cilostazol with PFA-100(R) and Multiplate(R) whole blood aggregometer in vitro, ex vivo and FeCl3-induced thrombosis models in vivo. Thromb Res. 2011; 127:565–70.27. Satoh K, Fukasawa I, Kanemaru K, Yoda S, Kimura Y, Inoue O, et al. Platelet aggregometry in the presence of PGE(1) provides a reliable method for cilostazol monitoring. Thromb Res. 2012; 130:616–21.
Article28. Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, Kimura Y, et al. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000; 59:347–56.
Article29. Angiolillo DJ. Antiplatelet therapy in diabetes: efficacy and limitations of current treatment strategies and future directions. Diabetes Care. 2009; 32:531–40.
Article30. Ajjan R, Storey RF, Grant PJ. Aspirin resistance and diabetes mellitus. Diabetologia. 2008; 51:385–90.
Article31. Fateh-Moghadam S, Plockinger U, Cabeza N, Htun P, Reuter T, Ersel S, et al. Prevalence of aspirin resistance in patients with type 2 diabetes. Acta Diabetol. 2005; 42:99–103.
Article32. Gachet C, Aleil B. Testing antiplatelet therapy. Eur Heart J Suppl. 2008; 10(suppl_A):A28–34.
Article33. Lordkipanidze M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007; 28:1702–8.
Article34. Rizzo M, Corrado E, Patti AM, Rini GB, Mikhailidis DP. Cilostazol and atherogenic dyslipidemia: a clinically relevant effect? Expert Opin Pharmacother. 2011; 12:647–55.
Article35. Hong S, Nam M, Little BB, Paik S, Lee K, Woo J, et al. Randomized control trial comparing the effect of cilostazol and aspirin on changes in carotid intima-medial thickness. Heart Vessels. 2019; 34:1758–68.
Article36. Kim BJ, Kwon SU, Park JH, Kim YJ, Hong KS, Wong LK, et al. Cilostazol versus aspirin in ischemic stroke patients with high-risk cerebral hemorrhage: subgroup analysis of the PICASSO trial. Stroke. 2020; 51:931–7.
Article37. Hsieh CJ, Wang PW. Effect of cilostazol treatment on adiponectin and soluble CD40 ligand levels in diabetic patients with peripheral arterial occlusion disease. Circ J. 2009; 73:948–54.
Article38. O’Donnell ME, Badger SA, Sharif MA, Makar RR, McEneny J, Young IS, et al. The effects of cilostazol on exerciseinduced ischaemia-reperfusion injury in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2009; 37:326–35.
Article39. Kim MA, Kim CJ, Seo JB, Chung WY, Kim SH, Zo JH, et al. The effect of aspirin on C-reactive protein in hypertensive patients. Clin Exp Hypertens. 2011; 33:47–52.
Article40. Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. Am J Cardiovasc Drugs. 2003; 3:117–38.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful sequential desensitization in a patient with drug hypersensitivity to three kinds of antiplatelet agents
- Effects of Antiplatelet Drugs in Cardiovascular Prevention
- The Effect of Cilostazol on Stent Thrombosis After Drug-Eluting Stent Implantation
- Effect of Antiplatelets in Diabetic Peripheral Vasculopathy: Comparison by Ankle-Brachial Index and Peak Wave Velocity
- Spontaneous Spinal Epidural Hematoma in a Patient on Cilostazol