Ann Pediatr Endocrinol Metab.
2022 Mar;27(1):37-43. 10.6065/apem.2142110.055.
Response to growth hormone according to provocation test results in idiopathic short stature and idiopathic growth hormone deficiency
- Affiliations
-
- 1Department of Pediatrics, Pusan National University Children’s Hospital, Yangsan, Korea
- 2Department of Pediatrics, Kosin University Gospel Hospital, Busan, Korea
- 3Department of Pediatrics, Pusan National University Hospital, Busan, Korea
- 4Department of Pediatrics, Ilsin Christian Hospital, Busan, Korea
- 5Department of Pediatrics, Inje University Haeundae Paik Hospital, Busan, Korea
- 6Department of Pediatrics, Inje University Busan Paik Hospital, Busan, Korea
- 7LG Chem, Ltd., Seoul, Korea
- 8Department of Pediatrics, Dong-A University Hospital, Busan, Korea
- KMID: 2528196
- DOI: http://doi.org/10.6065/apem.2142110.055
Abstract
- Purpose
To investigate growth response in children with either idiopathic short stature (ISS) or growth hormone (GH) deficiency (GHD).
Methods
The data of prepubertal GHD or ISS children treated using recombinant human GH were obtained from the LG Growth Study database. GHD children were further divided into partial and complete GHD groups. Growth response and factors predicting growth response after 1 and 2 years of GH treatment were investigated.
Results
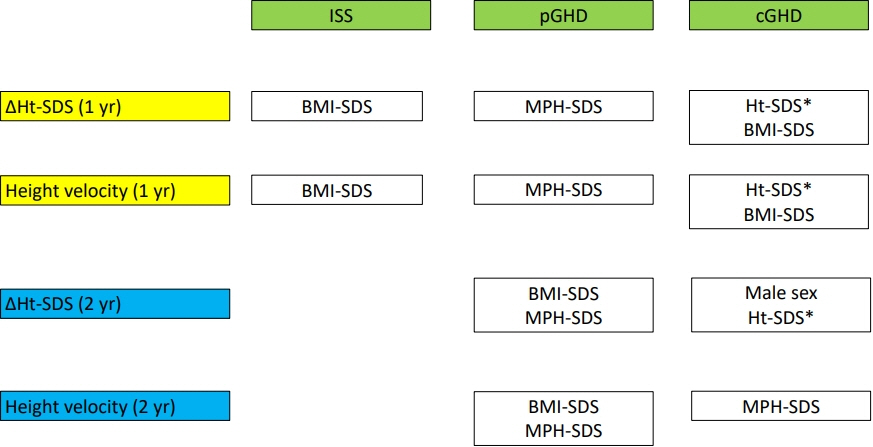
This study included 692 children (98 with ISS, 443 partial GHD, and 151 complete GHD). After 1 year, changes in height standard deviation score (ΔHt-SDS) were 0.78, 0.83, and 0.96 in ISS, partial GHD, and complete GHD, respectively. Height velocity (HV) was 8.72, 9.04, and 9.52 cm/yr in ISS, partial GHD, and complete GHD, respectively. ΔHt-SDS and HV did not differ among the 3 groups. Higher initial body mass index standard deviation score (BMI-SDS) and midparental height standard deviation score (MPH-SDS) were predictors for better growth response after 1 year in ISS and the partial GHD group, respectively. In the complete GHD group, higher Ht-SDS and BMI-SDS predicted better growth response after 1 year. After 2 years of GH treatment, higher BMI-SDS and MPH-SDS predicted a better growth outcome in the partial GHD group, and higher MPH-SDS was a predictor of good growth response in complete GHD.
Conclusion
Clinical characteristics and growth response did not differ among groups. Predictors of growth response differed among the 3 groups, and even in the same group, a higher GH dose would be required when poor response is predicted.
Keyword
Figure
Reference
-
References
1. Patel L, Clayton PE. Predicting response to growth hormone treatment. Indian J Pediatr. 2012; 79:229–37.2. Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment. Growth Horm IGF Res. 2009; 19:1–11.3. Cho WK, Ahn MB, Kim EY, Cho KS, Jung MH, Suh BK. Predicting first-year growth in response to growth hormone treatment in prepubertal Korean children with idiopathic growth hormone deficiency: analysis of data from the LG Growth Study Database. J Korean Med Sci. 2020; 35:e151.4. Hou L, Liang Y, Wu W, Lin HH, Luo XP, Ying YQ. Comparison of the efficacy and safety of recombinant human growth hormone in treating idiopathic short stature and growth hormone deficiency in children. Growth Horm IGF Res. 2020; 53-54:101331.5. Al Shaikh A, Daftardar H, Alghamdi AA, Jamjoom M, Awidah S, Ahmed ME, et al. Effect of growth hormone treatment on children with idiopathic short stature (ISS), idiopathic growth hormone deficiency (IGHD), small for gestational age (SGA), and Turner syndrome (TS) in a tertiary care center. Acta Biomed. 2020; 91:29–40.6. Chung S, Yoo JH, Choi JH, Rhie YJ, Chae HW, Kim JH, et al. Design of the long-term observational cohort study with recombinant human growth hormone in Korean children: LG Growth Study. Ann Pediatr Endocrinol Metab. 2018; 23:43–50.7. Rhie YJ, Yoo JH, Choi JH, Chae HW, Kim JH, Chung S, et al. Long-term safety and effectiveness of growth hormone therapy in Korean children with growth disorders: 5-year results of LG Growth Study. PLoS One. 2019; 14:e0216927.8. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.9. Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem. 2012; 45:16–21.10. Grelich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford (CA): Stanford University Press;1959.11. Van den Broeck J, Hering P, Van de Lely A, Hokken-Koelega A. Interpretative difficulties with growth hormone provocative retesting in childhood-onset growth hormone deficiency. Horm Res. 1999; 51:1–9.12. Ghigo E, Bellone J, Aimaretti G, Bellone S, Loche S, Cappa M, et al. Reliability of provocative tests to assess growth hormone secretory status. Study in 472 normally growing children. J Clin Endocrinol Metab. 1996; 81:3323–7.13. Fisker S, Jorgensen JO, Christiansen JS. Variability in growth hormone stimulation tests. Growth Horm IGF Res. 1998; 8 Suppl A:31–5.14. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor-i treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-i deficiency. Horm Res Paediatr. 2016; 86:361–97.15. Hanew K, Utsumi A. The role of endogenous GHRH in arginine-, insulin-, clonidine- and l-dopa-induced GH release in normal subjects. Eur J Endocrinol. 2002; 146:197–202.16. Hilczer M, Smyczynska J, Lewinski A. Limitations of clinical utility of growth hormone stimulating tests in diagnosing children with short stature. Endocr Regul. 2006; 40:69–75.17. Jeong HR, Kwon EB, Shim YS, Lee HS, Hwang JS. Comparative study of growth hormone treatment in children with idiopathic short stature and growth hormone deficiency. Curr Drug Metab. 2015; 16:940–6.18. Van den Broeck J, Arends N, Hokken-Koelega A. Growth response to recombinant human growth hormone (GH) in children with idiopathic growth retardation by level of maximum GH peak during GH stimulation tests. Horm Res. 2000; 53:267–73.19. Schena L, Meazza C, Pagani S, Paganelli V, Bozzola E, Tinelli C, et al. Efficacy of long-term growth hormone therapy in short non-growth hormone-deficient children. J Pediatr Endocrinol Metab. 2017; 30:197–201.20. Kim SA, Choe YR, Yang EM, Kim CJ. Comparison of growth hormone treatment in patients with idiopathic short stature and idiopathic growth hormone deficiency. Chonnam Med J. 2014; 50:63–6.21. Isojima T, Hasegawa T, Yokoya S, Tanaka T. The response to growth hormone treatment in prepubertal children with growth hormone deficiency in Japan: comparing three consecutive years of treatment data of The Foundation for Growth Science in Japan between the 1990s and 2000s. Endocr J. 2017; 64:851–8.22. Cardoso DF, Martinelli CE Jr, Campos VC, Gomes ES, Rocha IE, Oliveira CR, et al. Comparison between the growth response to growth hormone (GH) therapy in children with partial GH insensitivity or mild GH deficiency. Arq Bras Endocrinol Metabol. 2014; 58:23–9.23. Straetemans S, Roelants M, Thomas M, Rooman R, De Schepper J. Reference curve for the first-year growth response to growth hormone treatment in prepubertal children with idiopathic growth hormone deficiency: validation of the KIGS first-year growth response curve using the Belgian Register for the Study of Growth and Puberty Problems. Horm Res Paediatr. 2014; 81:343–9.24. Hawcutt DB, Bellis J, Price V, Povall A, Newland P, Richardson P, et al. Growth hormone prescribing and initial BMI SDS: Increased biochemical adverse effects and costs in obese children without additional gain in height. PLoS One. 2017; 12:e0181567.25. Esen I, Demirel F, Tepe D, Kara O, Koc N. The association between growth response to growth hormone and baseline body composition of children with growth hormone deficiency. Growth Horm IGF Res. 2013; 23:196–9.26. Gjikopulli A, Grimci L, Kollcaku L, Tomori S, Cullufi P, Hoxha P, et al. Final height in children with idiopathic growth hormone deficiency treated with growth hormone: Albanian experience. Curr Health Sci J. 2015; 41:22–8.27. Cutfield W, Lindberg A, Albertsson Wikland K, Chatelain P, Ranke MB, Wilton P. Final height in idiopathic growth hormone deficiency : the KIGS experience. KIGS International Board. Acta Paediatr Suppl. 1999; 88:72–5.28. Cutfield WS, Albert BB. Growth hormone treatment for idiopathic short stature. Pediatr Endocrinol Rev. 2018; 16:113–22.29. Cole TJ, Hindmarsh PC, Dunger DB. Growth hormone (GH) provocation tests and the response to GH treatment in GH deficiency. Arch Dis Child. 2004; 89:1024–7.30. Bright GM, Julius JR, Lima J, Blethen SL. Growth hormone stimulation test results as predictors of recombinant human growth hormone treatment outcomes: preliminary analysis of the national cooperative growth study database. Pediatrics. 1999; 104:1028–31.31. Collett-Solberg PF, Jorge AAL, Boguszewski MCS, Miller BS, Choong CSY, Cohen P, et al. Growth hormone therapy in children; research and practice – a review. Growth Horm IGF Res. 2019; 44:20–32.32. Sävendahl L, Blankenstein O, Oliver I, Christesen HT, Lee P, Pedersen BT, et al. Gender influences short-term growth hormone treatment response in children. Horm Res Paediatr. 2012; 77:188–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short Stature and Growth Hormone Therapy
- Overnight Growth Hormone Secretions and Sleep Patterns in Idiopathic Short Stature Children
- Comparison of Growth Hormone Treatment in Patients with Idiopathic Short Stature and Idiopathic Growth Hormone Deficiency
- Growth Hormone Therapy in Short Stature Children
- The Effects of Growth Hormone on Carbohydrate and Lipid Metabolism