Blood Res.
2022 Mar;57(1):34-40. 10.5045/br.2021.2021127.
Prophylaxis for invasive fungal infection in pediatric patients with allogeneic hematopoietic stem cell transplantation
- Affiliations
-
- 1Universidad Icesi, Facultad de Ciencias de la Salud, Cali, Colombia
- 2Fundación Valle del Lili, Departamento Materno-infantil, Servicio de Infectología Pediátrica, Cali, Colombia
- 3Fundación Valle del Lili, Departamento Materno-infantil, Unidad de Trasplante de Médula Ósea, Cali, Colombia
- 4Fundación Valle del Lili, Centro de Investigaciones Clínicas (CIC), Cali, Colombia
- KMID: 2527495
- DOI: http://doi.org/10.5045/br.2021.2021127
Abstract
- Background
Antifungal prophylaxis is recommended for hematopoietic stem cell transplantation (HSCT) to decrease the incidence of invasive fungal infections (IFI). This study aimed to compare the two groups of antifungal prophylaxis in pediatric patients undergoing allogeneic HSCT.
Methods
This observational, analytic, retrospective cohort study compared the incidence of IFI with antifungal prophylaxis with voriconazole vs. other antifungals in the first 100 days after allogeneic HSCT in patients aged <18 years between 2012 and 2018. The statistical analysis included univariate and multivariate analyses and determination of the cumulative incidence of invasive fungal infection by the Kaplan‒Meier method using STATA 14 statistical software.
Results
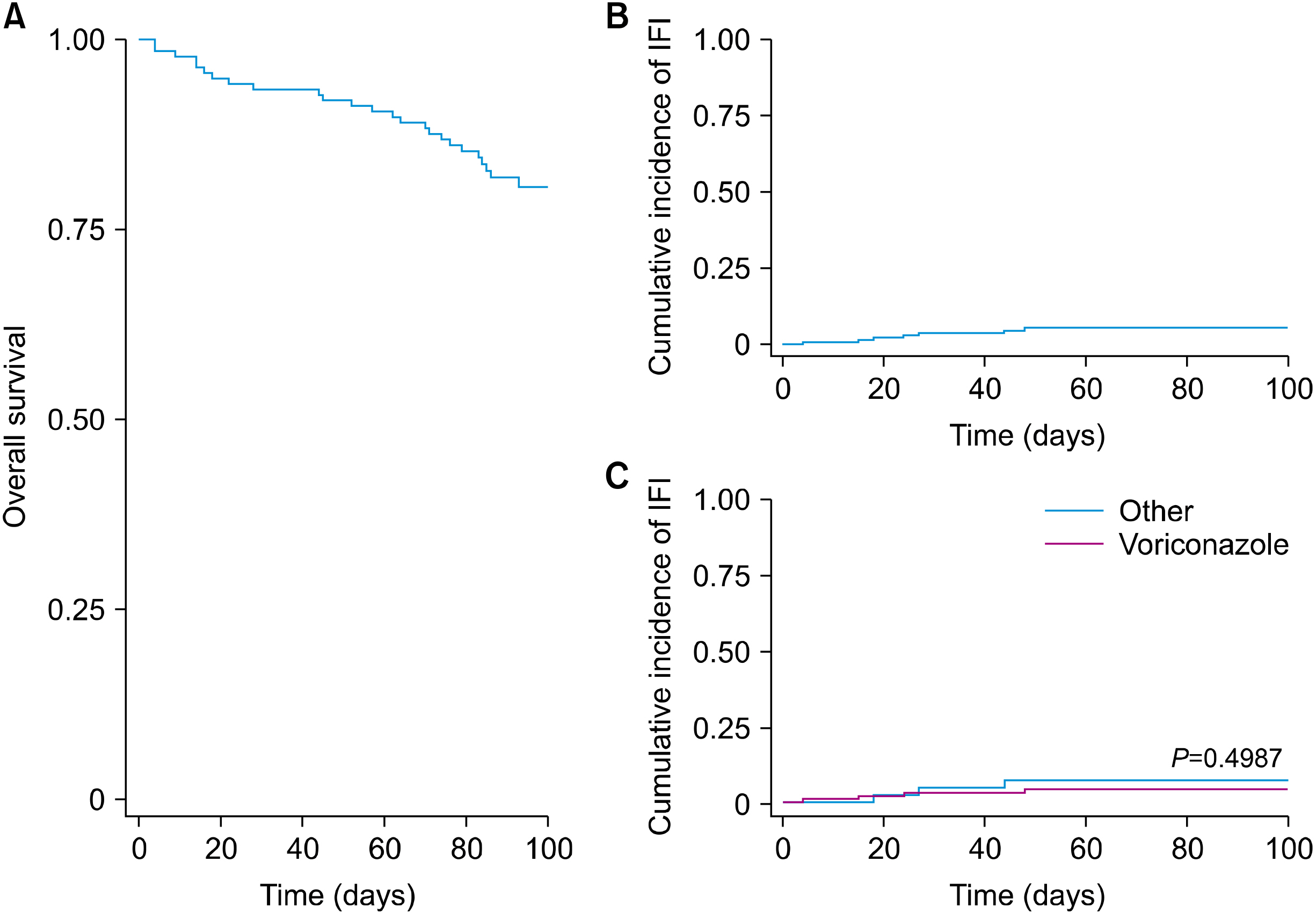
A total of 139 allogeneic HSCT were performed. The principal diagnosis was acute leukemia (63%). The 75% had haploidentical donors, and 50% used an antifungal in the month before transplantation. Voriconazole (69%) was the most frequently administered antifungal prophylaxis. The cumulative incidence of IFI was 5% (7 cases). Of the patients with IFIs, four began prophylaxis with voriconazole, one with caspofungin, and one with fluconazole. Additionally, six were possible cases, one was proven (Candida parapsilosis), and 1/7 died.
Conclusion
There were no differences in the incidence of IFI between patients who received prophylaxis with voriconazole and other antifungal agents.
Keyword
Figure
Reference
-
1. Badiee P, Hashemizadeh Z. 2014; Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res. 139:195–204. PMID: 24718393. PMCID: PMC4001330.2. Ramos JT, Francisco L, Daoud Z. 2016; Invasive fungal infections in children: similarities and differences with adults. Rev Esp Quimioter. 29(Suppl 1):59–65. PMID: 27608317.3. Cruz-Contreras D. 2016; Aspergilosis invasiva en el paciente que recibe trasplante alogénico de células progenitoras hematopoyéticas: epidemiología , diagnóstico, profilaxis y tratamiento. Rev Hematol Mex. 17:262–7.4. Omrani AS, Almaghrabi RS. 2017; Complications of hematopoietic stem transplantation: fungal infections. Hematol Oncol Stem Cell Ther. 10:239–44. DOI: 10.1016/j.hemonc.2017.05.013. PMID: 28636889.
Article5. Schwartz KL, Sheffield H, Richardson SE, Sung L, Morris SK. 2015; Invasive fusariosis: a single pediatric center 15-year experience. J Pediatric Infect Dis Soc. 4:163–70. DOI: 10.1093/jpids/pit080. PMID: 26407418.
Article6. Hazar V, Karasu GT, Uygun V, et al. 2019; Risks and outcomes of invasive fungal infections in pediatric allogeneic hematopoietic stem cell transplant recipients receiving fluconazole prophylaxis: a multicenter cohort study by the Turkish Pediatric Bone Marrow Transplantation Study Group. Med Mycol. 57:161–70. DOI: 10.1093/mmy/myy015. PMID: 29608706.
Article7. Hovi L, Saarinen-Pihkala UM, Vettenranta K, Saxen H. 2000; Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant. 26:999–1004. DOI: 10.1038/sj.bmt.1702654. PMID: 11100280.
Article8. Czyżewski K, Styczyński J, Giebel S, et al. 2019; Age-dependent determinants of infectious complications profile in children and adults after hematopoietic cell transplantation: lesson from the nationwide study. Ann Hematol. 98:2197–211. DOI: 10.1007/s00277-019-03755-2. PMID: 31321454. PMCID: PMC6700048.
Article9. Choi JK, Cho SY, Yoon SS, et al. 2017; Epidemiology and risk factors for invasive fungal diseases among allogeneic hematopoietic stem cell transplant recipients in Korea: results of "RISK" Study. Biol Blood Marrow Transplant. 23:1773–9. DOI: 10.1016/j.bbmt.2017.06.012. PMID: 28668492.
Article10. Ullmann AJ, Lipton JH, Vesole DH, et al. 2007; Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 356:335–47. DOI: 10.1056/NEJMoa061098. PMID: 17251530.
Article11. Sano H, Kobayashi R, Hori D, et al. 2018; Prophylactic administration of voriconazole with two different doses for invasive fungal infection in children and adolescents with acute myeloid leukemia. J Microbiol Immunol Infect. 51:260–6. DOI: 10.1016/j.jmii.2016.05.002. PMID: 27329132.
Article12. Molina JR, Serrano J, Sánchez-García J, et al. 2012; Voriconazole as primary antifungal prophylaxis in children undergoing allo-SCT. Bone Marrow Transplant. 47:562–7. DOI: 10.1038/bmt.2011.111. PMID: 21572466.
Article13. Marr KA, Crippa F, Leisenring W, et al. 2004; Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 103:1527–33. DOI: 10.1182/blood-2003-08-2644. PMID: 14525770.
Article14. Mariotti J, De Philippis C, Bramanti S, et al. 2019; Caspofungin for primary antifungal prophylaxis after T-cell-replete haploidentical stem cell transplantation with post-transplant cyclophosphamide. Eur J Haematol. 102:357–67. DOI: 10.1111/ejh.13214. PMID: 30672611. PMCID: PMC7163667.
Article15. Rosanova MT, Voto C, Mussini MS, et al. 2018; Uso de posaconazol en niños: experiencia en un hospital pediátrico de alta complejidad. Arch Argent Pediatr. 116:e451–4. DOI: 10.5546/aap.2018.e451.
Article16. Pana ZD, Kourti M, Vikelouda K, et al. 2018; Voriconazole antifungal prophylaxis in children with malignancies: a nationwide study. J Pediatr Hematol Oncol. 40:22–6. DOI: 10.1097/MPH.0000000000000926. PMID: 28816795.
Article17. Omer AK, Ziakas PD, Anagnostou T, et al. 2013; Risk factors for invasive fungal disease after allogeneic hematopoietic stem cell trans-plantation: a single center experience. Biol Blood Marrow Transplant. 19:1190–6. DOI: 10.1016/j.bbmt.2013.05.018. PMID: 23747459.
Article18. De Pauw B, Walsh TJ, Donnelly JP, et al. 2008; Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) C. Clin Infect Dis. 46:1813–21. DOI: 10.1086/588660. PMID: 18462102. PMCID: PMC2671227.
Article19. L Kandaurava S, S Baslyk K, A Migas A, et al. 2020; Comparative study of prophylaxis with high and low doses of Voriconazole in children with malignancy. Curr Med Mycol. 6:27–34. DOI: 10.18502/cmm.6.4.5331. PMID: 34195457. PMCID: PMC8226053. PMID: 9f572bc1f306441288b05208de240928.20. Asociación Española de Pediatría. 2021. Voriconazol. Pediamecum. Asociación Española de Pediatría;Madrid, Spain: at https://www.aeped.es/pediamecum/generatepdf/api?n=83577. Accessed January 5, 2021.21. Mendoza-Palomar N, Soques E, Benitez-Carabante MI, et al. 2020; Low-dose liposomal amphotericin B for antifungal prophylaxis in paediatric allogeneic haematopoietic stem cell transplantation. J Antimicrob Chemother. 75:2264–71. DOI: 10.1093/jac/dkaa149. PMID: 32335674.
Article22. Bacigalupo A, Ballen K, Rizzo D, et al. 2009; Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 15:1628–33. DOI: 10.1016/j.bbmt.2009.07.004. PMID: 19896087. PMCID: PMC2861656.
Article23. Kanda Y, Hyo R, Yamashita T, et al. 2006; Effect of blood cyclosporine concentration on the outcome of hematopoietic stem cell transplantation from an HLA-matched sibling donor. Am J Hematol. 81:838–44. DOI: 10.1002/ajh.20710. PMID: 16888784.
Article24. Al-Homsi AS, Roy TS, Cole K, Feng Y, Duffner U. 2015; Post-transplant high-dose cyclophosphamide for the prevention of graft-versus-host disease. Biol Blood Marrow Transplant. 21:604–11. DOI: 10.1016/j.bbmt.2014.08.014. PMID: 25240817.
Article25. Medina D, Estacio M, Rosales M, Manzi E. 2020; Haploidentical stem cell transplant with post-transplantation cyclophosphamide and mini-dose methotrexate in children. Hematol Oncol Stem Cell Ther. 13:208–13. DOI: 10.1016/j.hemonc.2020.01.003. PMID: 32224144.
Article26. Holler E, Greinix H, Zeiser R. 2019; Acute graft-versus-host disease. In: Carreras E, Dufour C, Mohty M, Kröger N, eds. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. 7th ed. Cham, Switzerland:. Springer,. 323–30. DOI: 10.1007/978-3-030-02278-5_43. PMID: 32091805.27. Rowlings PA, Przepiorka D, Klein JP, et al. 1997; IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 97:855–64. DOI: 10.1046/j.1365-2141.1997.1112925.x. PMID: 9217189.
Article28. Hahn T, Sucheston-Campbell LE, Preus L, et al. 2015; Establishment of definitions and review process for consistent adjudication of cause-specific mortality after allogeneic unrelated-donor hemato-poietic cell transplantation. Biol Blood Marrow Transplant. 21:1679–86. DOI: 10.1016/j.bbmt.2015.05.019. PMID: 26028504. PMCID: PMC4537799.
Article29. Carlesse F, Daudt LE, Seber A, et al. 2019; A consensus document for the clinical management of invasive fungal diseases in pediatric patients with hematologic cancer and/or undergoing hematopoietic stem cell transplantation in Brazilian medical centers. Braz J Infect Dis. 23:395–409. DOI: 10.1016/j.bjid.2019.09.005. PMID: 31738887.
Article30. Panichella M, Epelbaum C, Rosanova MT, et al. 2016; Infecciones fúngicas en pacientes hemato-oncológicos pediátricos/fungal infections in pediatric hematology-oncology patients. Med Infant. 23:18–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Lung Ultrasound to Evaluate Invasive Fungal Diseases after Allogeneic Hematopoietic Stem Cell Transplantation
- Lung Ultrasound to Evaluate Invasive Fungal Diseases after Allogeneic Hematopoietic Stem Cell Transplantation
- Successful Allogeneic Hematopoietic Stem Cell Transplantation for a Patient with Very Severe Aplastic Anemia During Active Invasive Fungal Infection
- Successful Hematopoietic Stem Cell Transplantation in Myelodysplastic Syndrome with Invasive Fungal Infection: A Case Report
- Disseminated Aspergillosis following Allogeneic Hematopoietic Stem Cell Transplantation in an Acute Leukemic Patient who was Previously Treated for Invasive Aspergillosis