Blood Res.
2022 Mar;57(1):1-3. 10.5045/br.2022.2021134.
A call for vigilance: thrombotic thrombocytopenic syndrome caused by mRNA COVID-19 vaccine associated with muscle weakness
- Affiliations
-
- 1Department of Internal Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 2Department of Neurology, Sahmyook Medical Center, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 3Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University College of Medicine and Bundang Hospital, Seongnam, Korea
- KMID: 2527488
- DOI: http://doi.org/10.5045/br.2022.2021134
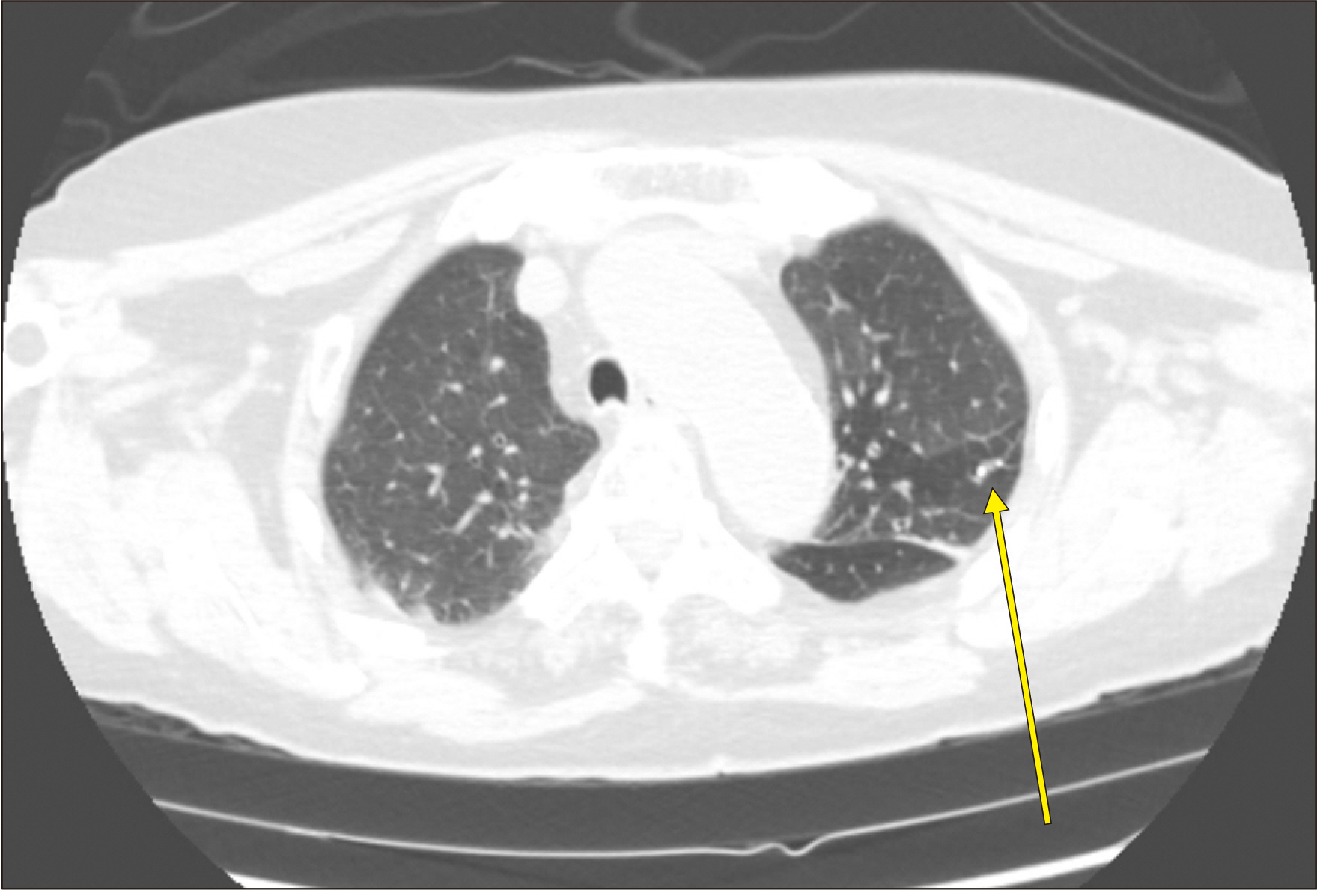
Figure
Reference
-
1. Zhu N, Zhang D, Wang W, et al. 2020; A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 382:727–33. DOI: 10.1056/NEJMoa2001017. PMID: 31978945. PMCID: PMC7092803.
Article2. Krause PR, Gruber MF. 2020; Emergency use authorization of covid vaccines-safety and efficacy follow-up considerations. N Engl J Med. 383:e107. DOI: 10.1056/NEJMp2031373. PMID: 33064383.3. Choi JK, Kim S, Kim SR, et al. 2021; Intracerebral hemorrhage due to thrombosis with thrombocytopenia syndrome after vaccination against COVID-19: the first fatal case in Korea. J Korean Med Sci. 36:e223. DOI: 10.3346/jkms.2021.36.e223. PMID: 34402235. PMCID: PMC8352786.
Article4. Weintraub K. 2021. Jan. 6. Death of Florida doctor after receiving COVID-19 vaccine under investigation. USA Today.5. Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. 2021; Thrombo-cytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 39:3329–32. DOI: 10.1016/j.vaccine.2021.04.054. PMID: 34006408. PMCID: PMC8086806.
Article6. Sangli S, Virani A, Cheronis N, et al. 2021; Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine. Ann Intern Med. 174:1480–2. DOI: 10.7326/L21-0244. PMID: 34181446. PMCID: PMC8251935.
Article7. Al-Maqbali JS, Al Rasbi S, Kashoub MS, et al. 2021; A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 days following a first dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Am J Case Rep. 22:e932946. DOI: 10.12659/AJCR.932946. PMID: 34117206. PMCID: PMC8212841.
Article8. Lunn MP, Cornblath DR, Jacobs BC, et al. 2021; COVID-19 vaccine and Guillain-Barré syndrome: let's not leap to associations. Brain. 144:357–60. DOI: 10.1093/brain/awaa444. PMID: 33313690. PMCID: PMC7799242.
Article9. Mack M, Nichols L, Guerrero DM. 2021; Rhabdomyolysis secondary to COVID-19 vaccination. Cureus. 13:e15004. DOI: 10.7759/cureus.15004. PMID: 34150372. PMCID: PMC8202440.
Article10. Thiele T, Ulm L, Holtfreter S, et al. 2021; Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 138:299–303. DOI: 10.1182/blood.2021012217. PMID: 33988688. PMCID: PMC8129797.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 vaccination–related cardiovascular complications
- Review of COVID-19 Vaccines and Their Evidence in Older Adults
- mRNA COVID-19 Vaccine-Associated Subserosal Eosinophilic Gastroenteritis: A Case Report
- Thrombotic Thrombocytopenic Purpura and Rhabdomyolysis Associated With Acute Renal Failure in Hypothyroidism
- Case Reports of Acute Transverse Myelitis Associated With mRNA Vaccine for COVID-19