J Stroke.
2022 Jan;24(1):138-147. 10.5853/jos.2021.01606.
Cost-Effectiveness of Endovascular Thrombectomy in Childhood Stroke: An Analysis of the Save ChildS Study
- Affiliations
-
- 1Department of Radiology, University Hospital, LMU Munich, Munich, Germany
- 2Department of Neuroradiology, Clinic for Radiology & Nuclear Medicine, University Hospital Basel, Switzerland
- 3Department of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 4Department of Neuroradiology, Alfried-Krupp Hospital, Essen, Germany
- 5Department of Neuroradiology, University Hospital Bonn, Bonn, Germany
- 6Department of Neuroradiology, Medical University of Innsbruck, Innsbruck, Austria.
- 7Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy
- 8Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- 9Division of Child Neurology, Department of Neurology, Stanford University, Stanford, CA, USA
- 10Department for Neuroradiology, University Hospital Marburg, Marburg, Germany
- 11Department of Neuroradiology, Klinikum Stuttgart, Stuttgart, Germany
- 12Department of Neuroradiology, Aachen University, Aachen, Germany
- 13Department of Radiology and Neuroradiology, University Hospital of Schleswig-Holstein, Kiel, Germany
- 14Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany
- 15Department of Neuroradiology, University Hospital of Cologne, Cologne, Germany
- 16Division of Neuroradiology and Musculoskeletal Radiology, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria
- 17Department for Neuroradiology, University Hospital Leipzig, Leipzig, Germany
- 18Department of Radiology, University Hospital Regensburg, Regensburg, Germany
- 19Institute of Neuroradiology, University Hospital Duesseldorf, Duesseldorf, Germany
- 20Department of Diagnostic and Interventional Neuroradiology, Hannover Medical School, Hannover, Germany
- 21Department of Neuroradiology, University Hospital Carl Gustav Carus, Dresden, Germany
- 22Department of Neurology, University Hospital, LMU Munich, Munich, Germany
- 23Institute of Diagnostic and Interventional Neuroradiology, University Hospital, LMU Munich, Munich, Germany
- KMID: 2525339
- DOI: http://doi.org/10.5853/jos.2021.01606
Abstract
- Background and Purpose
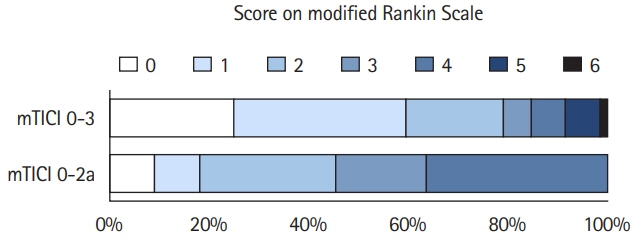
The Save ChildS Study demonstrated that endovascular thrombectomy (EVT) is a safe treatment option for pediatric stroke patients with large vessel occlusions (LVOs) with high recanalization rates. Our aim was to determine the long-term cost, health consequences and cost-effectiveness of EVT in this patient population.
Methods
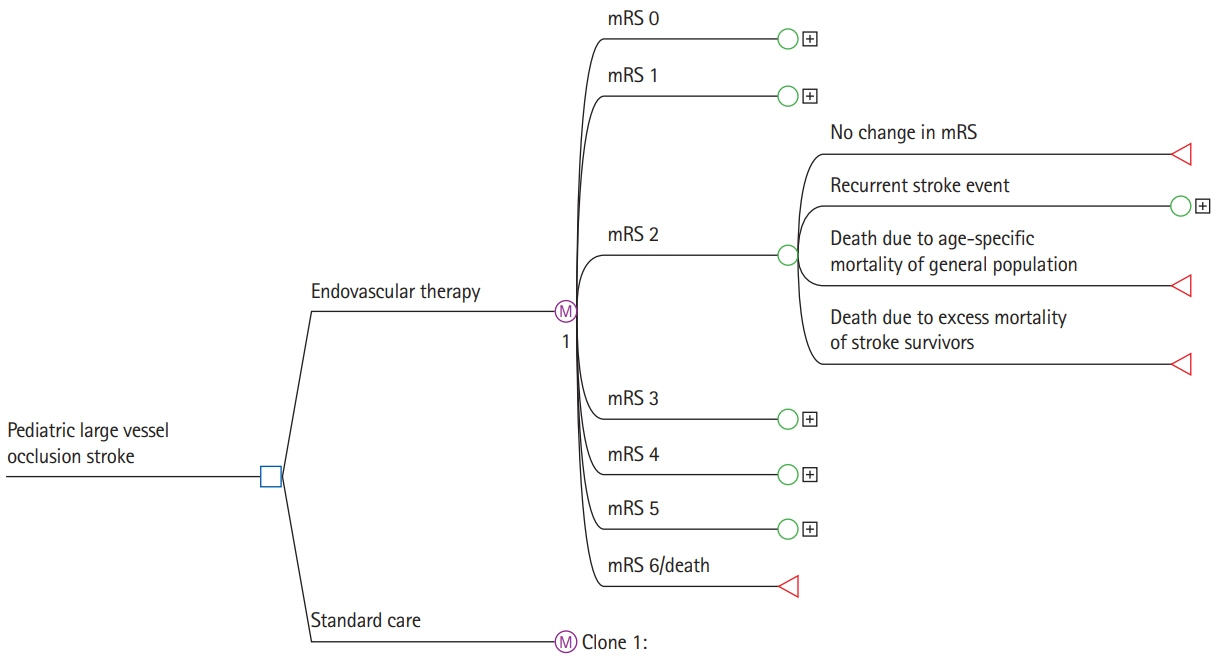
In this retrospective study, a decision-analytic Markov model estimated lifetime costs and quality-adjusted life years (QALYs). Early outcome parameters were based on the entire Save ChildS Study to model the EVT group. As no randomized data exist, the Save ChildS patient subgroup with unsuccessful recanalization was used to model the standard of care group. For modeling of lifetime estimates, pediatric and adult input parameters were obtained from the current literature. The analysis was conducted in a United States setting applying healthcare and societal perspectives. Probabilistic sensitivity analyses were performed. The willingness-to-pay threshold was set to $100,000 per QALY.
Results
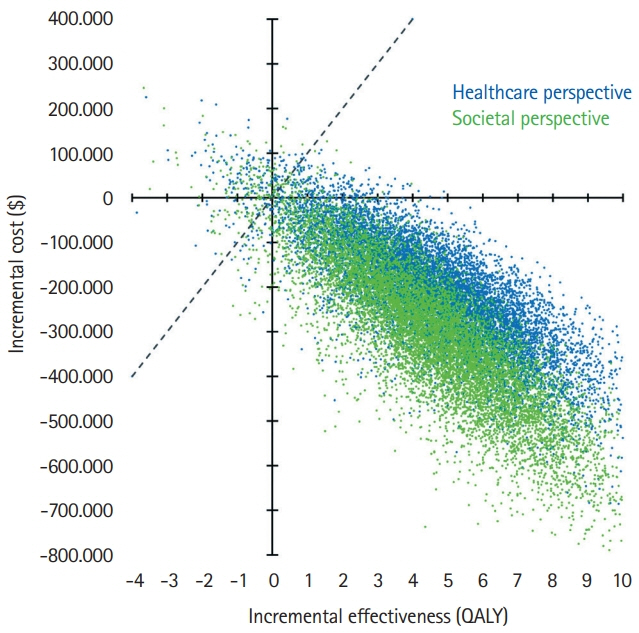
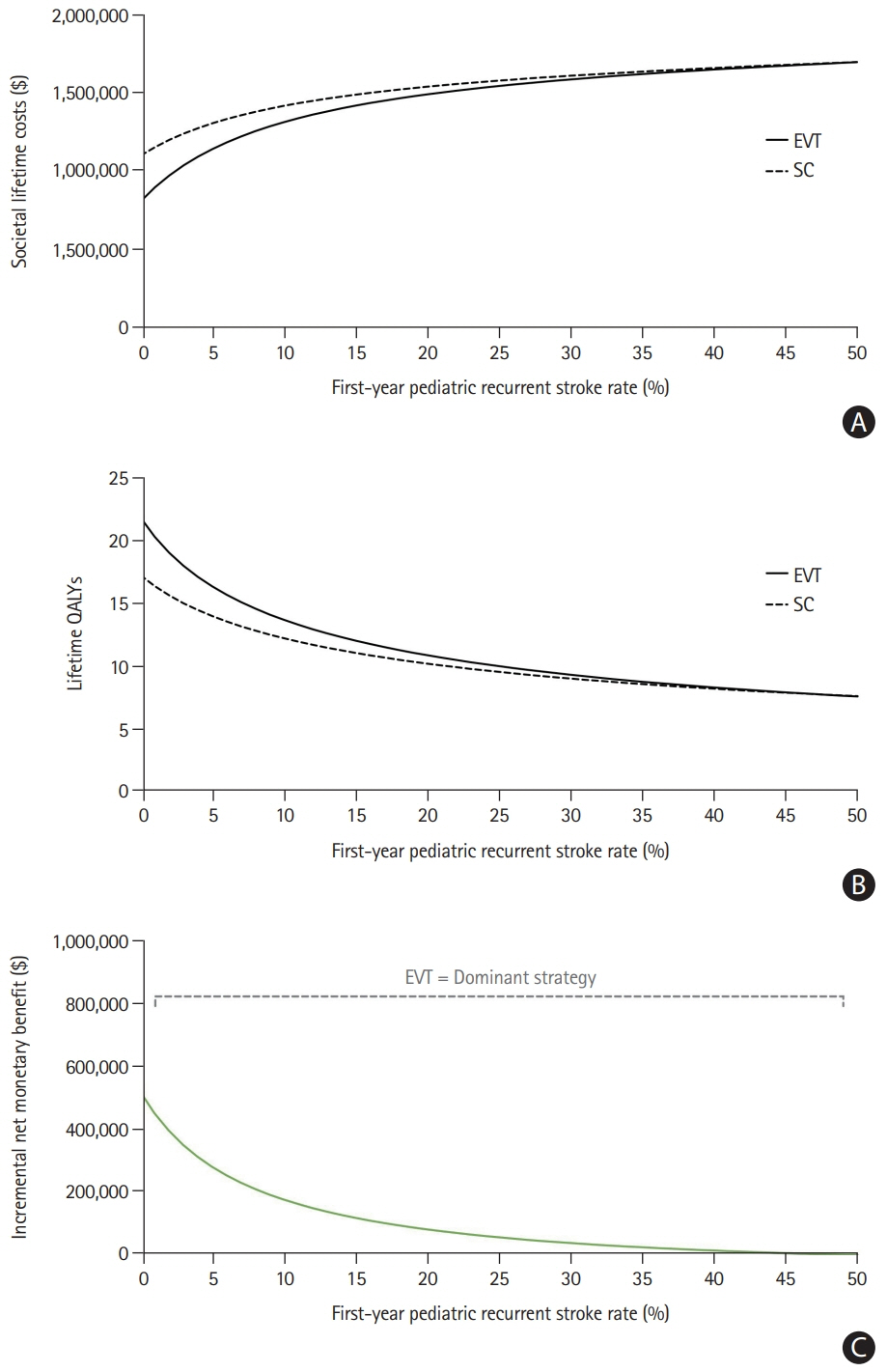
The model results yielded EVT as the dominant (cost-effective as well as cost-saving) strategy for pediatric stroke patients. The incremental effectiveness for the average age of 11.3 years at first stroke in the Save ChildS Study was determined as an additional 4.02 lifetime QALYs, with lifetime cost-savings that amounted to $169,982 from a healthcare perspective and $254,110 when applying a societal perspective. Acceptability rates for EVT were 96.60% and 96.66% for the healthcare and societal perspectives.
Conclusions
EVT for pediatric stroke patients with LVOs resulted in added QALY and reduced lifetime costs. Based on the available data in the Save ChildS Study, EVT is very likely to be a cost-effective treatment strategy for childhood stroke.
Keyword
Figure
Reference
-
References
1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics: 2021 update: a report from the American Heart Association. Circulation. 2021; 143:e254–e743.2. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.
Article3. Leppert MH, Campbell JD, Simpson JR, Burke JF. Cost-effectiveness of intra-arterial treatment as an adjunct to intravenous tissue-type plasminogen activator for acute ischemic stroke. Stroke. 2015; 46:1870–1876.
Article4. Ganesalingam J, Pizzo E, Morris S, Sunderland T, Ames D, Lobotesis K. Cost-utility analysis of mechanical thrombectomy using stent retrievers in acute ischemic stroke. Stroke. 2015; 46:2591–2598.
Article5. Aronsson M, Persson J, Blomstrand C, Wester P, Levin LÅ. Cost-effectiveness of endovascular thrombectomy in patients with acute ischemic stroke. Neurology. 2016; 86:1053–1059.
Article6. Kunz WG, Hunink MG, Sommer WH, Beyer SE, Meinel FG, Dorn F, et al. Cost-effectiveness of endovascular stroke therapy: a patient subgroup analysis from a US healthcare perspective. Stroke. 2016; 47:2797–2804.7. Shireman TI, Wang K, Saver JL, Goyal M, Bonafé A, Diener HC, et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke: results from the SWIFT-PRIME trial (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke). Stroke. 2017; 48:379–387.8. Jordan LC, Hillis AE. Challenges in the diagnosis and treatment of pediatric stroke. Nat Rev Neurol. 2011; 7:199–208.
Article9. Rivkin MJ, deVeber G, Ichord RN, Kirton A, Chan AK, Hovinga CA, et al. Thrombolysis in pediatric stroke study. Stroke. 2015; 46:880–885.
Article10. Amlie-Lefond C, Shaw DW, Cooper A, Wainwright MS, Kirton A, Felling RJ, et al. Risk of intracranial hemorrhage following intravenous tPA (tissue-type plasminogen activator) for acute stroke is low in children. Stroke. 2020; 51:542–548.
Article11. Sporns PB, Kemmling A, Hanning U, Minnerup J, Sträter R, Niederstadt T, et al. Thrombectomy in Childhood Stroke. J Am Heart Assoc. 2019; 8:e011335.
Article12. Sporns PB, Sträter R, Minnerup J, Wiendl H, Hanning U, Chapot R, et al. Feasibility, safety, and outcome of endovascular recanalization in childhood stroke: the Save ChildS Study. JAMA Neurol. 2020; 77:25–34.
Article13. Ellis C, McGrattan K, Mauldin P, Ovbiagele B. Costs of pediatric stroke care in the United States: a systematic and contemporary review. Expert Rev Pharmacoecon Outcomes Res. 2014; 14:643–650.
Article14. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016; 316:1093–1103.15. Luengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013; 44:2854–2861.16. Wolfe CD, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLoS Med. 2011; 8:e1001033.
Article17. Ganesh A, Luengo-Fernandez R, Wharton RM, Gutnikov SA, Silver LE, Mehta Z, et al. Time course of evolution of disability and cause-specific mortality after ischemic stroke: implications for trial design. J Am Heart Assoc. 2017; 6:e005788.
Article18. Pennlert J, Eriksson M, Carlberg B, Wiklund PG. Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke. 2014; 45:1839–1841.
Article19. Arias E, Xu J. United States life tables, 2018. Natl Vital Stat Rep. 2020; 69:1–45.20. Slot KB, Berge E, Sandercock P, Lewis SC, Dorman P, Dennis M, et al. Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke. 2009; 40:1585–1589.
Article21. Dawson J, Lees JS, Chang TP, Walters MR, Ali M, Davis SM, et al. Association between disability measures and healthcare costs after initial treatment for acute stroke. Stroke. 2007; 38:1893–1898.
Article22. Caro JJ, Huybrechts KF, Duchesne I. Management patterns and costs of acute ischemic stroke: an international study. For the Stroke Economic Analysis Group. Stroke. 2000; 31:582–590.23. Caro JJ, Huybrechts KF, Kelley HE. Predicting treatment costs after acute ischemic stroke on the basis of patient characteristics at presentation and early dysfunction. Stroke. 2001; 32:100–106.
Article24. Luengo-Fernandez R, Gray AM, Rothwell PM. Population-based study of determinants of initial secondary care costs of acute stroke in the United Kingdom. Stroke. 2006; 37:2579–2587.
Article25. Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol. 2009; 8:235–243.
Article26. Yoneda Y, Uehara T, Yamasaki H, Kita Y, Tabuchi M, Mori E. Hospital-based study of the care and cost of acute ischemic stroke in Japan. Stroke. 2003; 34:718–724.
Article27. Diringer MN, Edwards DF, Mattson DT, Akins PT, Sheedy CW, Hsu CY, et al. Predictors of acute hospital costs for treatment of ischemic stroke in an academic center. Stroke. 1999; 30:724–728.
Article28. Alvarez-Sabín J, Quintana M, Masjuan J, Oliva-Moreno J, Mar J, Gonzalez-Rojas N, et al. Economic impact of patients admitted to stroke units in Spain. Eur J Health Econ. 2017; 18:449–458.
Article29. Holloway RG, Witter DM Jr, Lawton KB, Lipscomb J, Samsa G. Inpatient costs of specific cerebrovascular events at five academic medical centers. Neurology. 1996; 46:854–860.30. Castonguay AC, Zaidat OO, Novakovic R, Nguyen TN, Taqi MA, Gupta R, et al. Influence of age on clinical and revascularization outcomes in the North American Solitaire Stent-Retriever Acute Stroke Registry. Stroke. 2014; 45:3631–3636.31. Beumer D, Rozeman AD, Lycklama À Nijeholt GJ, Brouwer PA, Jenniskens SF, Algra A, et al. The effect of age on outcome after intra-arterial treatment in acute ischemic stroke: a MR CLEAN pretrial study. BMC Neurol. 2016; 16:68.
Article32. Chambers MG, Koch P, Hutton J. Development of a decision-analytic model of stroke care in the United States and Europe. Value Health. 2002; 5:82–97.
Article33. Chaisinanunkul N, Adeoye O, Lewis RJ, Grotta JC, Broderick J, Jovin TG, et al. Adopting a patient-centered approach to primary outcome analysis of acute stroke trials using a utilityweighted modified Rankin scale. Stroke. 2015; 46:2238–2243.
Article34. Hallan S, Asberg A, Indredavik B, Widerøe TE. Quality of life after cerebrovascular stroke: a systematic study of patients’ preferences for different functional outcomes. J Intern Med. 1999; 246:309–316.
Article35. Gargano JW, Reeves MJ; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke. 2007; 38:2541–2548.36. Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, et al. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke. 2006; 37:2567–2572.
Article37. Naess H, Waje-Andreassen U, Thomassen L, Nyland H, Myhr KM. Health-related quality of life among young adults with ischemic stroke on long-term follow-up. Stroke. 2006; 37:1232–1236.
Article38. Kunz WG, Hunink MG, Dimitriadis K, Huber T, Dorn F, Meinel FG, et al. Cost-effectiveness of endovascular therapy for acute ischemic stroke: a systematic review of the impact of patient age. Radiology. 2018; 288:518–526.39. Sträter R, Becker S, von Eckardstein A, Heinecke A, Gutsche S, Junker R, et al. Prospective assessment of risk factors for recurrent stroke during childhood: a 5-year follow-up study. Lancet. 2002; 360:1540–1545.40. Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD, et al. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke. 2003; 34:1457–1463.41. Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke. 2004; 35:731–735.
Article42. Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011; 42:1489–1494.43. Consumer Price Index. U.S. Bureau of Labor Statistics. http://www.bls.gov/cpi. 2020. Accessed October 28, 2021.44. Current population survey. United States Census Bureau. https://www.census.gov/cps/data/cpstablecreator.html. 2019. Accessed October 28, 2021.45. Household data annual averages: 3. employment status of the civilian noninstitutional population by age, sex, and race. U.S. Bureau of Labor Statistics. https://www.bls.gov/cps/cpsaat03.pdf. 2020. Accessed October 28, 2021.46. Vyas MV, Hackam DG, Silver FL, Laporte A, Kapral MK. Lost productivity in stroke survivors: an econometrics analysis. Neuroepidemiology. 2016; 47:164–170.
Article47. Brown DL, Boden-Albala B, Langa KM, Lisabeth LD, Fair M, Smith MA, et al. Projected costs of ischemic stroke in the United States. Neurology. 2006; 67:1390–1395.
Article48. Tanaka H, Toyonaga T, Hashimoto H. Functional and occupational characteristics predictive of a return to work within 18 months after stroke in Japan: implications for rehabilitation. Int Arch Occup Environ Health. 2014; 87:445–453.
Article49. Saeki S, Ogata H, Okubo T, Takahashi K, Hoshuyama T. Factors influencing return to work after stroke in Japan. Stroke. 1993; 24:1182–1185.
Article50. Ferro JM, Crespo M. Prognosis after transient ischemic attack and ischemic stroke in young adults. Stroke. 1994; 25:1611–1616.
Article51. Hickenbottom SL, Fendrick AM, Kutcher JS, Kabeto MU, Katz SJ, Langa KM. A national study of the quantity and cost of informal caregiving for the elderly with stroke. Neurology. 2002; 58:1754–1759.
Article52. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014; 371:796–797.53. Bigi S, Dulcey A, Gralla J, Bernasconi C, Melliger A, Datta AN, et al. Feasibility, safety, and outcome of recanalization treatment in childhood stroke. Ann Neurol. 2018; 83:1125–1132.
Article54. Sporns PB, Psychogios MN, Straeter R, Hanning U, Minnerup J, Chapot R, et al. Clinical diffusion mismatch to select pediatric patients for embolectomy 6 to 24 hours after stroke: an analysis of the Save ChildS Study. Neurology. 2021; 96:e343–e351.55. Sporns PB, Straeter R, Minnerup J, Wiendl H, Hanning U, Chapot R, et al. Does device selection impact recanalization rate and neurological outcome?: an analysis of the Save ChildS Study. Stroke. 2020; 51:1182–1189.56. Sun LR, Harrar D, Drocton G, Castillo-Pinto C, Felling R, Carpenter JL, et al. Mechanical thrombectomy for acute ischemic stroke: considerations in children. Stroke. 2020; 51:3174–3181.57. Chabrier S, Ozanne A, Naggara O, Boulouis G, Husson B, Kossorotoff M. Hyperacute recanalization strategies and childhood stroke in the evidence age. Stroke. 2021; 52:381–384.
Article58. Sporns PB, Psychogios M. Thrombectomy in childhood stroke: important considerations in borderline indications. Stroke. 2020; 51:2890–2891.59. Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain. 2019; 142:1399–1407.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differences in Effectiveness among Devices for Endovascular Thrombectomy in Patients with Acute Ischemic Stroke
- Endovascular Treatment of Acute Ischemic Stroke
- Intravenous Thrombolysis and Endovascular Thrombectomy in Acute Ischemic Stroke with Minor Symptom
- Resurrection of Endovascular Thrombectomy for Posterior Circulation Stroke
- Endovascular Thrombectomy in Patients with Intracranial Atherosclerosis: Where Are We?