Intest Res.
2022 Jan;20(1):144-149. 10.5217/ir.2020.00041.
A case of autoimmune enteropathy with CTLA4 haploinsufficiency
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Kobe University Hospital, Kobe, Japan
- 2Department of Gastroenterology, Kakogawa Central City Hospital, Kakogawa, Japan
- 3Department of Pediatrics, Kobe University Graduate School of Medicine, Kobe, Japan
- 4Department of Public Health, Kobe University Graduate School of Health Sciences, Kobe, Japan
- 5Department of Pediatrics, Juntendo University Faculty of Medicine, Tokyo, Japan
- 6Department of Pediatrics and Developmental Biology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
- 7Department of Community Pediatrics, Perinatal and Maternal Medicine, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
- 8Department of Child Health and Development, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
- 9Center for Pediatric Allergy and Rheumatology, KKR Sapporo Medical Center, Sapporo, Japan
- KMID: 2525081
- DOI: http://doi.org/10.5217/ir.2020.00041
Abstract
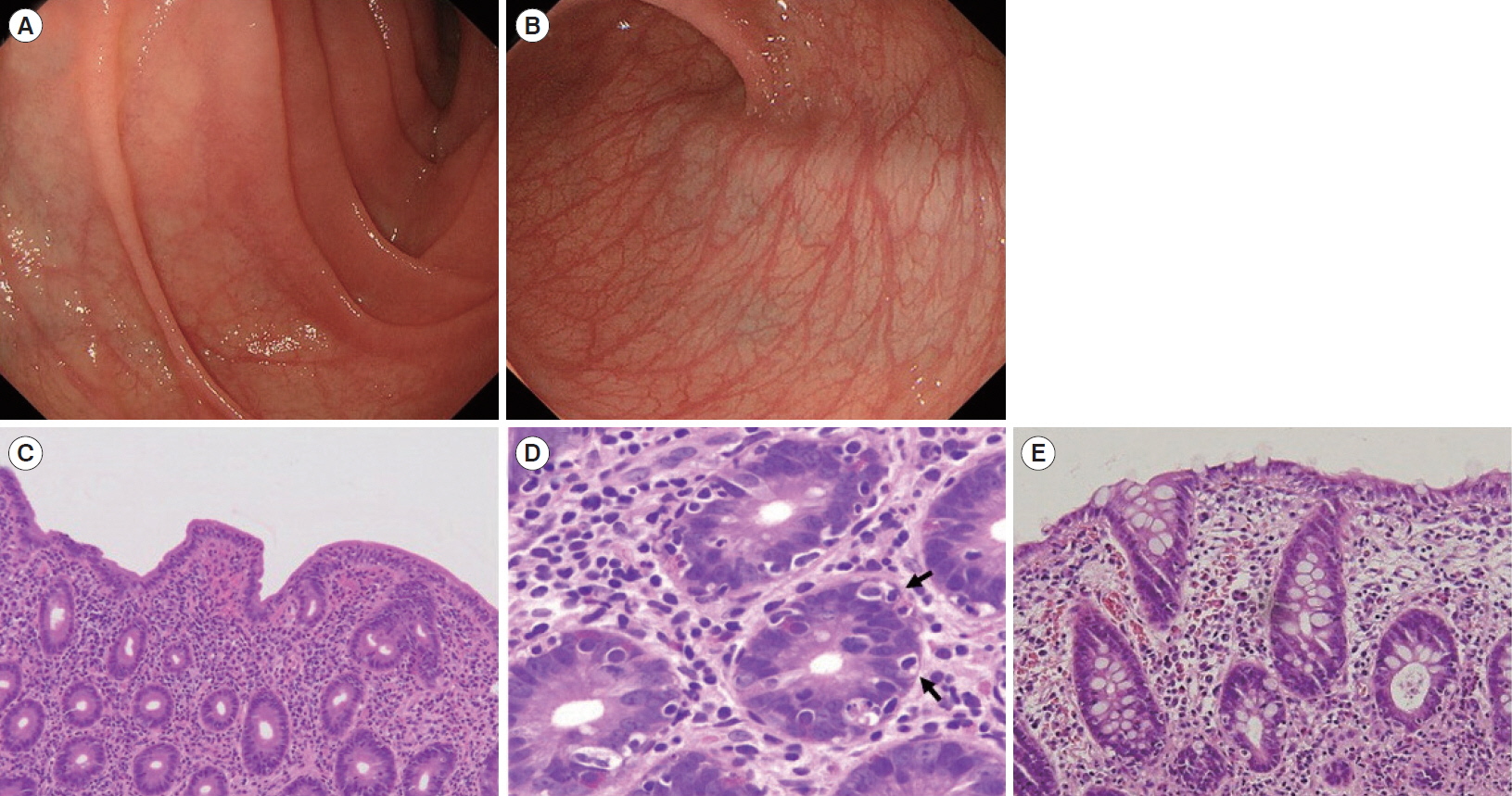
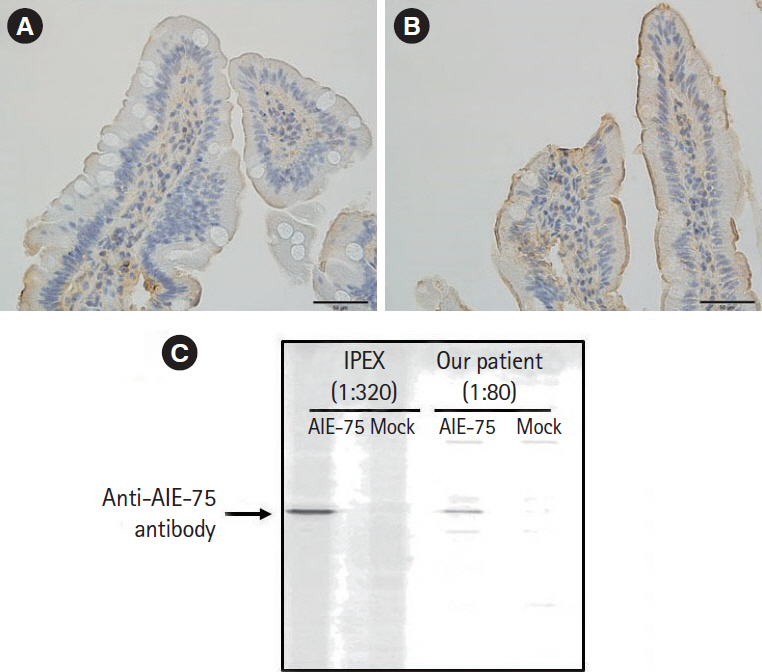
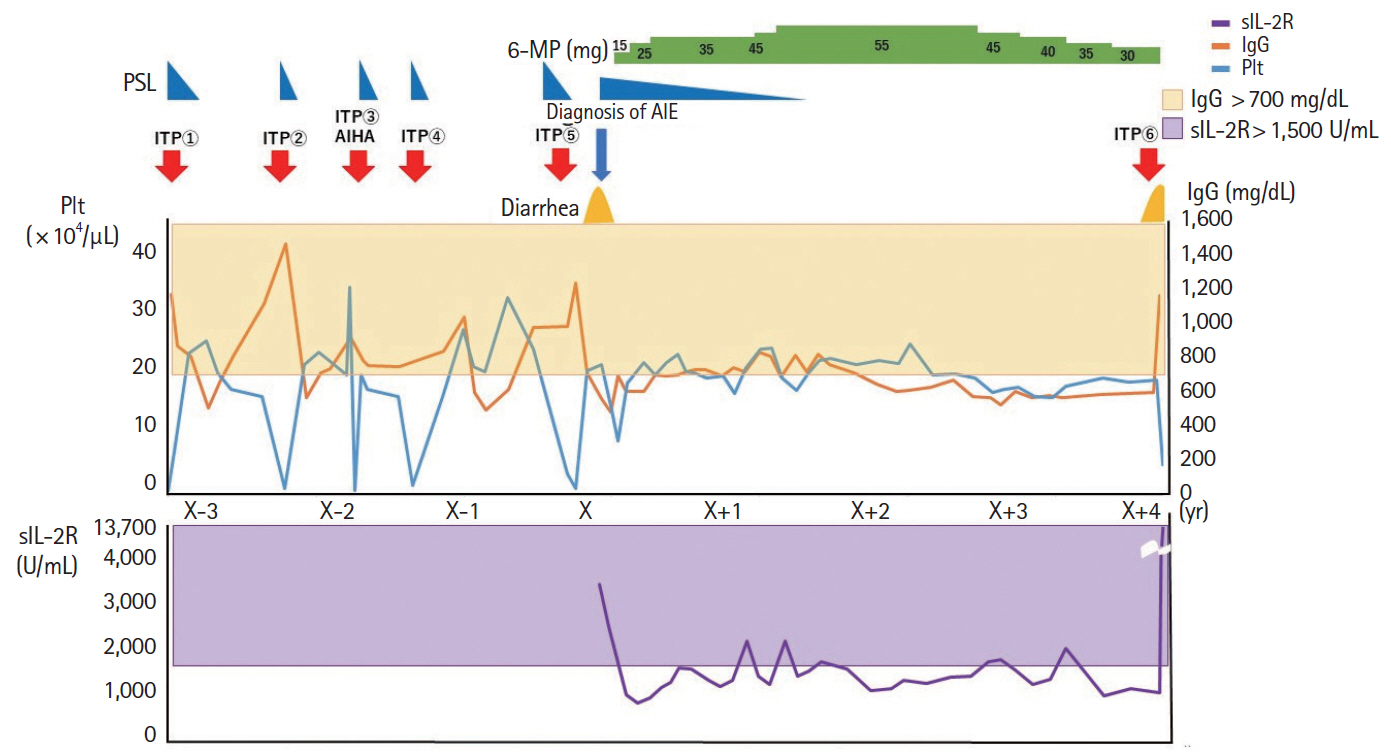
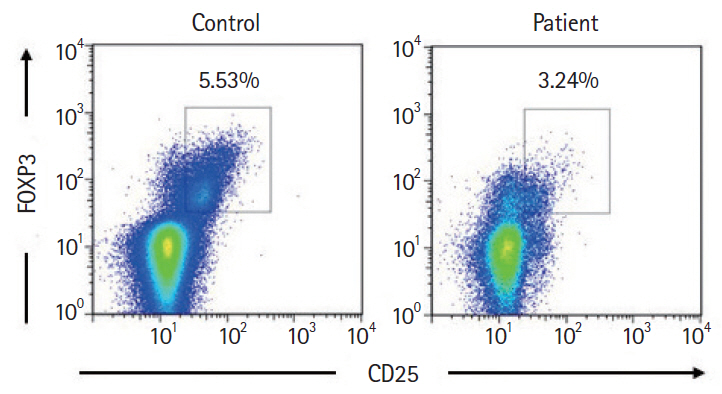
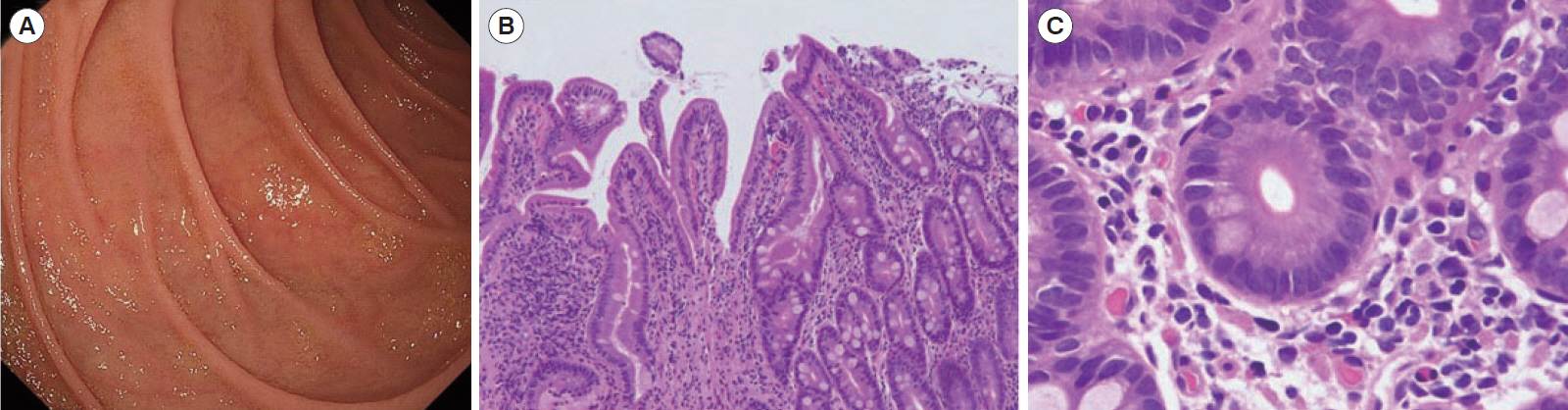
- Autoimmune enteropathy (AIE) is a rare disease, characterized by intractable diarrhea, villous atrophy of the small intestine, and the presence of circulating anti-enterocyte autoantibodies. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, and mutations in FOXP3, which is a master gene of regulatory T cells (Tregs), are major causes of AIE. Recent studies have demonstrated that mutations in other Treg-associated genes, such as CD25 and CTLA4, show an IPEX-like phenotype. We present the case of a 13-year-old girl with CTLA4 haploinsufficiency, suffering from recurrent immune thrombocytopenic purpura and intractable diarrhea. We detected an autoantibody to the AIE-related 75 kDa antigen (AIE-75), a hallmark of the IPEX syndrome, in her serum. She responded well to a medium dose of prednisolone and a controlled dose of 6-mercaptopurine (6-MP), even after the cessation of prednisolone administration. Serum levels of the soluble interleukin-2 receptor and immunoglobulin G (IgG) were useful in monitoring disease activity during 6-MP therapy. In conclusion, autoimmune-mediated mechanisms, similar to the IPEX syndrome, may be involved in the development of enteropathy in CTLA4 haploinsufficiency. Treatment with 6-MP and monitoring of disease activity using serum levels of soluble interleukin-2 receptor and IgG is suggested for such cases.
Figure
Reference
-
1. Unsworth DJ, Walker-Smith JA. Autoimmunity in diarrhoeal disease. J Pediatr Gastroenterol Nutr. 1985; 4:375–380.
Article2. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and nonself. Nat Immunol. 2005; 6:345–352.
Article3. Barzaghi F, Amaya Hernandez LC, Neven B, et al. Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: an international multicenter retrospective study. J Allergy Clin Immunol. 2018; 141:1036–1049.4. Kobayashi I, Imamura K, Kubota M, et al. Identification of an autoimmune enteropathy-related 75-kilodalton antigen. Gastroenterology. 1999; 117:823–830.
Article5. Kobayashi I, Kubota M, Yamada M, et al. Autoantibodies to villin occur frequently in IPEX, a severe immune dysregulation, syndrome caused by mutation of FOXP3. Clin Immunol. 2011; 141:83–89.
Article6. Schubert D, Bode C, Kenefeck R, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014; 20:1410–1416.
Article7. Kuehn HS, Ouyang W, Lo B, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014; 345:1623–1627.8. Gambineri E, Ciullini Mannurita S, Hagin D, et al. Clinical, immunological, and molecular heterogeneity of 173 patients with the phenotype of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Front Immunol. 2018; 9:2411.
Article9. Singhi AD, Goyal A, Davison JM, Regueiro MD, Roche RL, Ranganathan S. Pediatric autoimmune enteropathy: an entity frequently associated with immunodeficiency disorders. Mod Pathol. 2014; 27:543–553.
Article10. Kanegane H, Hoshino A, Okano T, et al. Flow cytometry-based diagnosis of primary immunodeficiency diseases. Allergol Int. 2018; 67:43–54.
Article11. Schwab C, Gabrysch A, Olbrich P, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol. 2018; 142:1932–1946.
Article12. Greil C, Roether F, La Rosée P, Grimbacher B, Duerschmied D, Warnatz K. Rescue of cytokine storm due to hlh by hemoadsorption in a CTLA4-deficient patient. J Clin Immunol. 2017; 37:273–276.
Article13. Masia R, Peyton S, Lauwers GY, Brown I. Gastrointestinal biopsy findings of autoimmune enteropathy: a review of 25 cases. Am J Surg Pathol. 2014; 38:1319–1329.14. Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006; 212:131–148.
Article15. Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008; 322:271–275.
Article16. Chida N, Kobayashi I, Takezaki S, et al. Disease specificity of anti-tryptophan hydroxylase-1 and anti-AIE-75 autoantibodies in APECED and IPEX syndrome. Clin Immunol. 2015; 156:36–42.
Article17. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with antiCTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015; 13:211.
Article18. Adler BL, Pezhouh MK, Kim A, et al. Histopathological and immunophenotypic features of ipilimumab-associated colitis compared to ulcerative colitis. J Intern Med. 2018; 283:568–577.
Article19. Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimise azathioprine therapy in patients with inflammatory bowel disease. Gut. 2001; 48:642–646.
Article20. D’Halluin PN, Tribut O, Branger B, et al. RBC 6-TGN and hematological parameters in patients with Crohn’s disease treated by azathioprine. Gastroenterol Clin Biol. 2005; 29:1264–1269.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Autoimmune Enteropathy Treated with Corticosteroid and FK506
- A Case of Primary Sjogren's Syndrome with Protein-losing Enteropathy
- Polymorphism of CTLA4 Gene in Schizophrenia
- CTLA4 expression profiles and their association with clinical outcomes of breast cancer: a systemic review
- A case of systemic lupus erythematosus with chylothorax, chronic interstitial cystitis and protein-losing enteropathy