Cancer Res Treat.
2022 Jan;54(1):109-117. 10.4143/crt.2020.1329.
Induction Chemotherapy as a Prognostication Index and Guidance for Treatment of Locally Advanced Head and Neck Squamous Cell Carcinoma: The Concept of Chemo-Selection (KCSG HN13-01)
- Affiliations
-
- 1Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 4Department of Hematology-Oncology, Ajou University Hospital, Suwon, Korea
- 5Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- 6Department of Hemato-Oncology, Keimyung University Dongsan Medical Center, Daegu, Korea
- 7Department of Hematology and Oncology, Ewha Womans University Hospital, Seoul, Korea
- 8Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 9Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 10Department of Hematology-Oncology, Yeungnam University Medical Center, Daegu, Korea
- 11Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 12Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea
- 13Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 14Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 15Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Korea
- KMID: 2524592
- DOI: http://doi.org/10.4143/crt.2020.1329
Abstract
- Purpose
Certain patient subgroups who do not respond to induction chemotherapy (IC) show inherent chemoresistance in locally advanced head and neck squamous cell carcinoma (LA-HNSCC). This study aimed to assess the prognostic value of IC, and role of IC in guiding the selection of a definitive locoregional therapy.
Materials and Methods
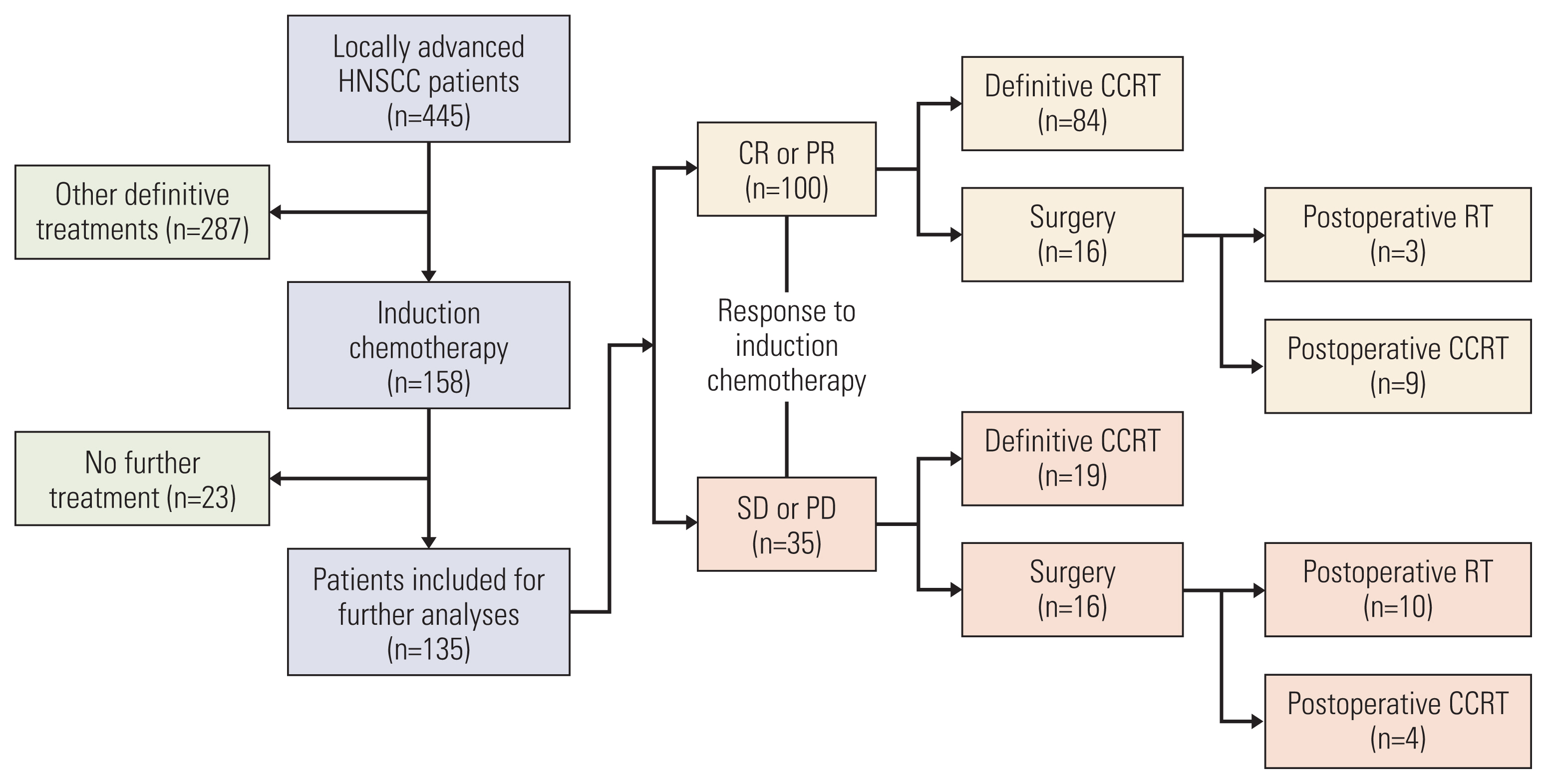
Out of the 445 patients in multi-institutional LA-HNSCC cohort, 158 (36%) receiving IC were enrolled. The study outcome was to assess overall survival (OS) through IC responsiveness and its role to select subsequent treatments.
Results
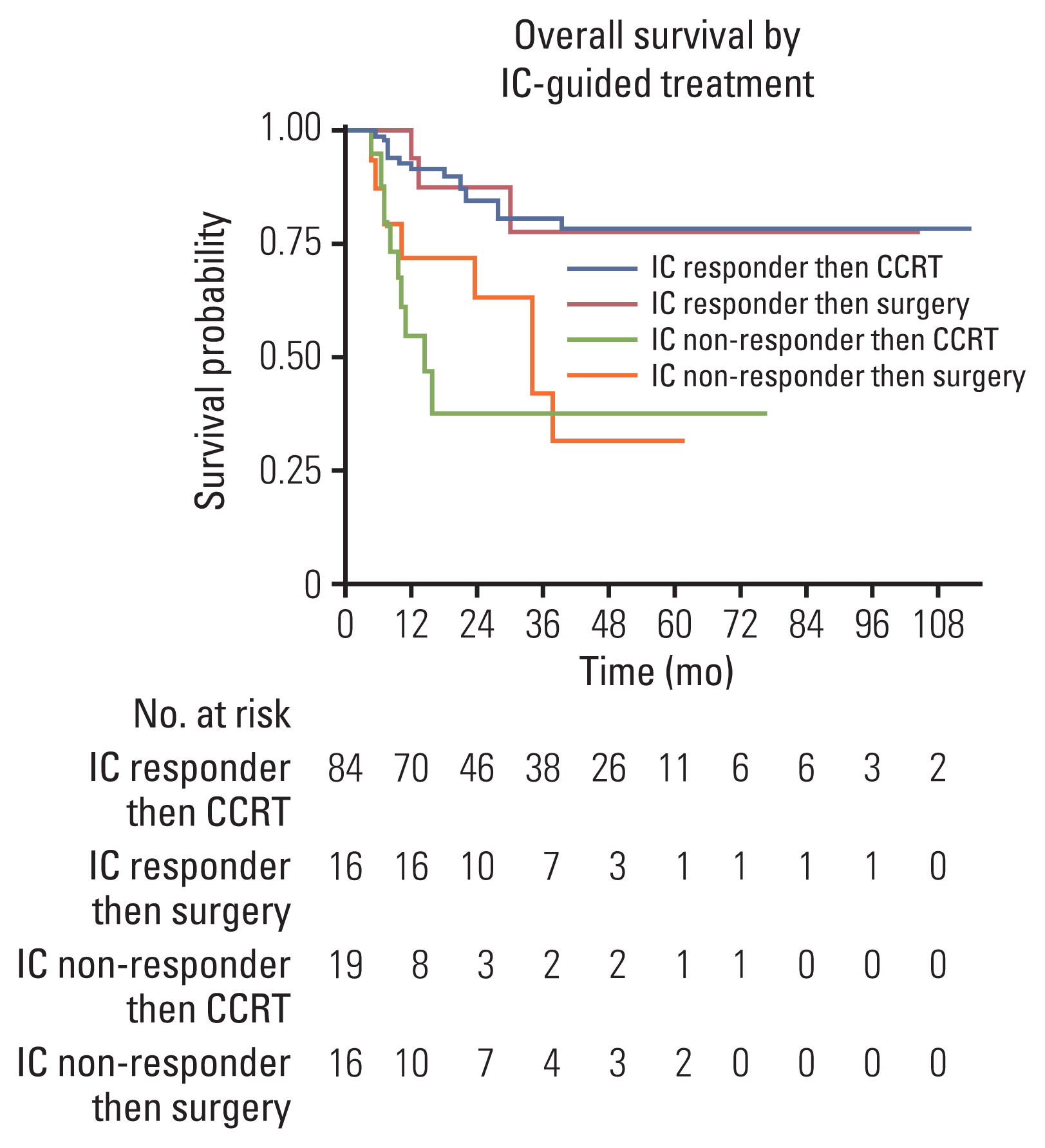
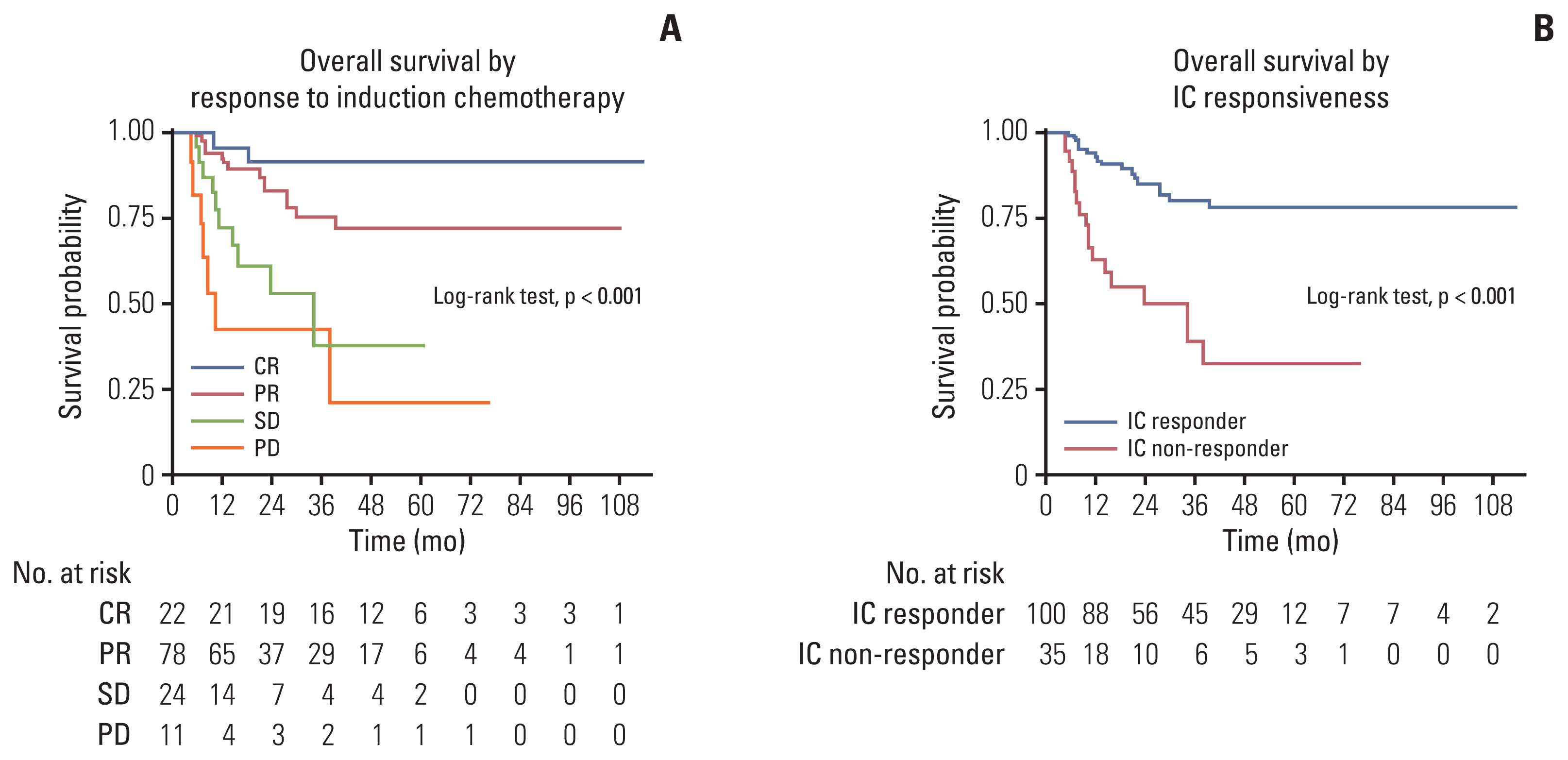
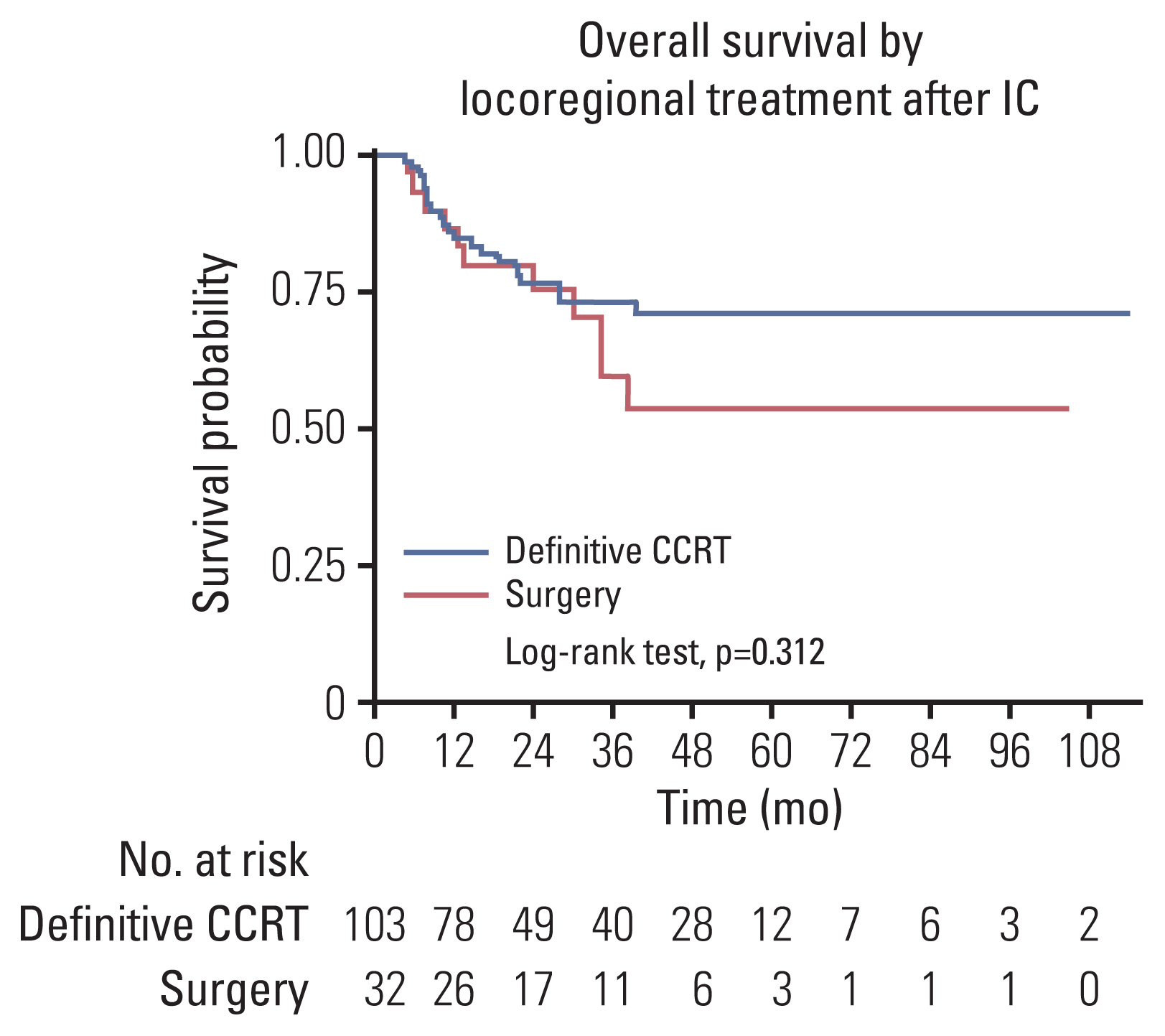
Among 135 patients who completed subsequent treatment following IC, 74% responded to IC (complete response in 17% and partial response in 58%). IC-non-responders showed 4.5 times higher risk of mortality than IC-responders (hazard ratio, 4.52; 95% confidence interval, 2.32 to 8.81; p < 0.001). Among IC-responders, 84% subsequently received definitive concurrent chemoradiotherapy (CCRT) and OS was not differed by surgery or CCRT (p=0.960). Regarding IC-non-responders, 54% received CCRT and 46% underwent surgery, and OS was poor in CCRT (24-month survival rate of 38%) or surgery (24-month survival rate of 63%).
Conclusion
Response to IC is a favorable prognostic factor. For IC-responders, either surgery or CCRT achieved similar survival probabilities. For IC-non-responder, multidisciplinary approach was warranted reflecting patients’ preference, morbidity, and prognosis.
Keyword
Figure
Reference
-
References
1. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016; 91:386–96.
Article2. Lee YG, Kang EJ, Keam B, Choi JH, Kim JS, Park KU, et al. Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective cohort study (KCSG HN13-01). BMC Cancer. 2020; 20:813.
Article3. Pignon JP, le Maitre A, Maillard E, Bourhis J. MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009; 92:4–14.
Article4. Brizel DM, Prosnitz RG, Hunter S, Fisher SR, Clough RL, Downey MA, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004; 58:1418–23.
Article5. Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014; 32:2735–43.
Article6. Zhang L, Jiang N, Shi Y, Li S, Wang P, Zhao Y. Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta-analysis. Sci Rep. 2015; 5:10798.
Article7. Haddad RI, Posner M, Hitt R, Cohen EE, Schulten J, Lefebvre JL, et al. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck: role, controversy, and future directions. Ann Oncol. 2018; 29:1130–40.
Article8. Gregoire V, Lefebvre JL, Licitra L, Felip E. EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21(Suppl 5):v184–6.9. Janoray G, Pointreau Y, Garaud P, Chapet S, Alfonsi M, Sire C, et al. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, +/− docetaxel for larynx preservation. J Natl Cancer Inst. 2016; 108:djv368.
Article10. Kim R, Hahn S, Shin J, Ock CY, Kim M, Keam B, et al. The effect of induction chemotherapy using docetaxel, cisplatin, and fluorouracil on survival in locally advanced head and neck squamous cell carcinoma: a meta-analysis. Cancer Res Treat. 2016; 48:907–16.
Article11. Kiong KL, de Souza NN, Sultana R, Iyer NG. Meta-analysis of induction chemotherapy as a selection marker for chemoradiation in the head and neck. Laryngoscope. 2018; 128:1594–601.
Article12. Ensley JF, Jacobs JR, Weaver A, Kinzie J, Crissman J, Kish JA, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984; 54:811–4.
Article13. Hong WK, O’Donoghue GM, Sheetz S, Fofonoff S, Dorman EB, Welch J, et al. Sequential response patterns to chemotherapy and radiotherapy in head and neck cancer: potential impact of treatment in advanced laryngeal cancer. Prog Clin Biol Res. 1985; 201:191–7.14. Department of Veterans Affairs Laryngeal Cancer Study Group. Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991; 324:1685–90.
Article15. Lefebvre JL, Andry G, Chevalier D, Luboinski B, Collette L, Traissac L, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012; 23:2708–14.
Article16. Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009; 101:498–506.
Article17. Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013; 14:257–64.
Article18. Geoffrois L, Martin L, Garaud P, De Raucourt D, Miny J, Maingon P, et al. Induction docetaxel platinum 5-FU (TPF) followed by cetuximab-radiotherapy (cetux-RT) versus concurrent chemo-radiotherapy (CT/RT) in patients with N2b/c-N3 non operated stage III–IV squamous cell cancer of the head and neck (SCCHN): results of the GORTEC 2007-02 phase III randomized trial. J Clin Oncol. 2016; 34(15 Suppl):6000.
Article19. Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017; 35:490–7.20. Ernani V, Saba NF. Oral cavity cancer: risk factors, pathology, and management. Oncology. 2015; 89:187–95.
Article21. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007; 357:1705–15.
Article22. Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011; 12:153–9.
Article23. Blanchard P, Bourhis J, Lacas B, Posner MR, Vermorken JB, Cruz Hernandez JJ, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013; 31:2854–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Organ Preservation for the Management of Locally Advanced Head and Neck Cancer
- Chemotherapy of Head and Neck Cancer
- Chemo-radiation Therapy for Locally Advanced Non-Small Cell Lung Cancer
- Induction chemotherapy in patients with locally-advanced head and neck squamous cell carcinoma: docetaxel and cisplatin
- Comparison of Radiation Therapy and Combined Chemotherapy and Radiation Therapy for Locally Advanced Head and Neck Cancer