Blood Res.
2021 Dec;56(4):342-345. 10.5045/br.2021.2021104.
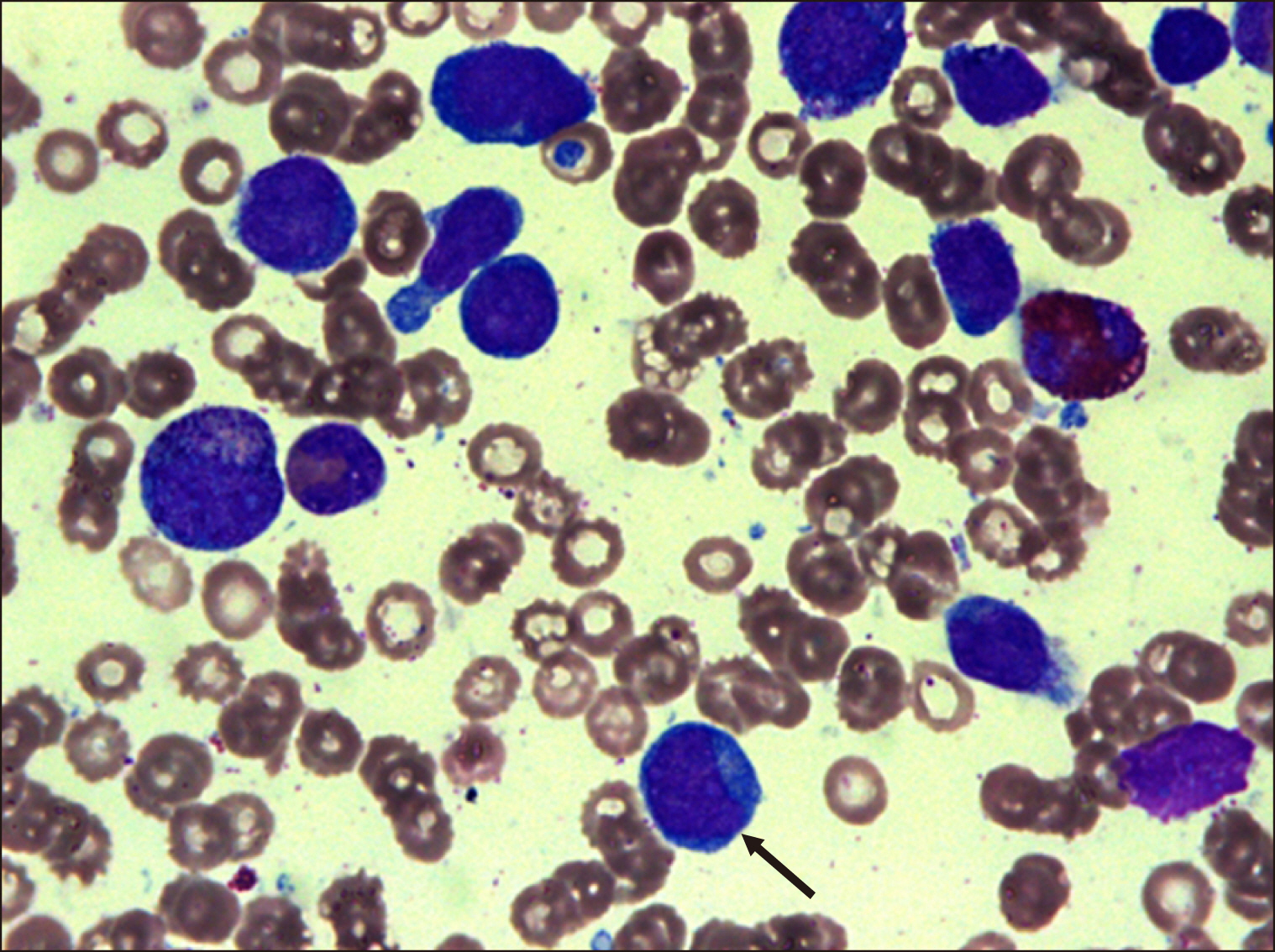
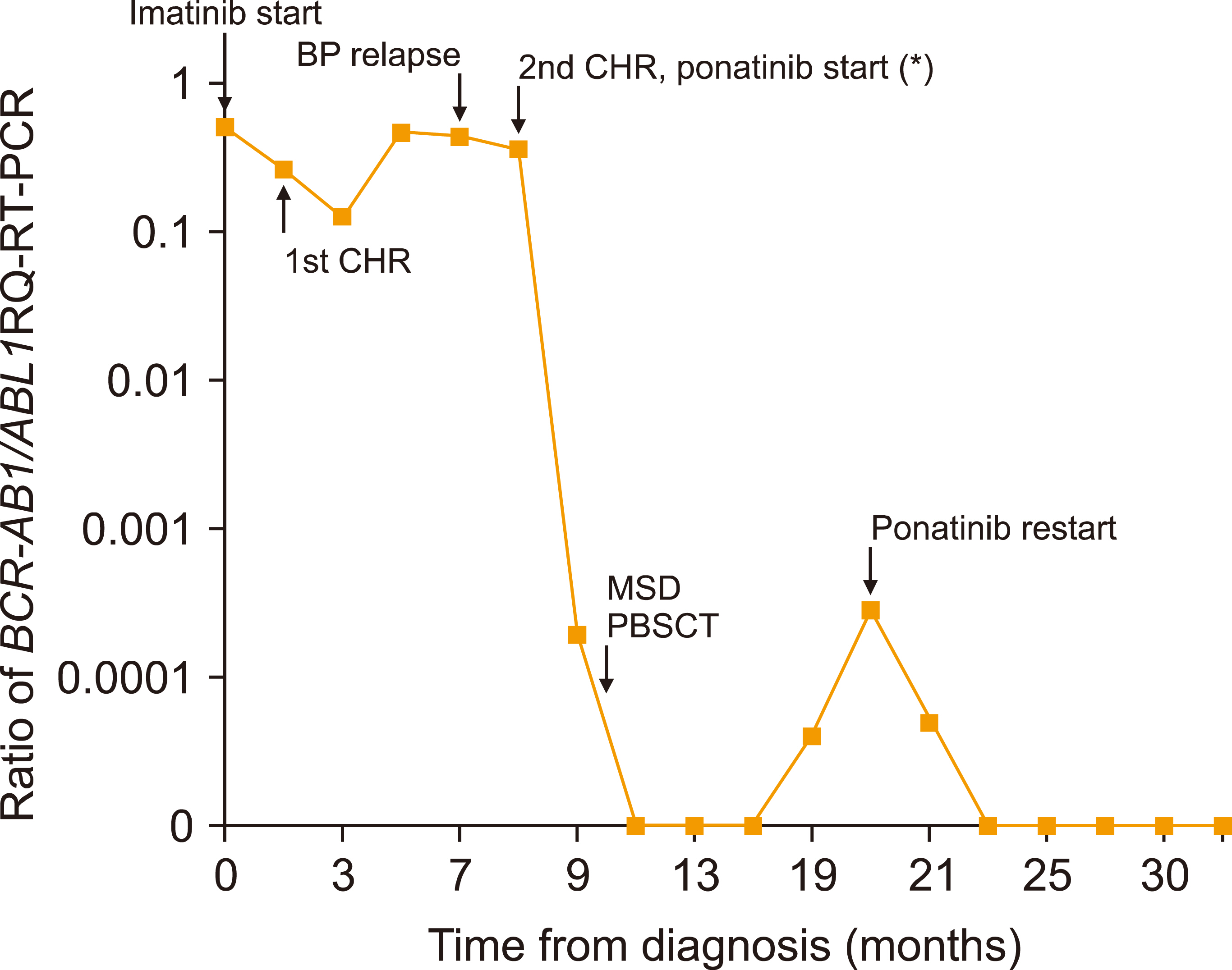
Efficacy of ponatinib prior to and after allogeneic hematopoietic stem cell transplantation in an adolescent with chronic myeloid leukemia in blast phase
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2524097
- DOI: http://doi.org/10.5045/br.2021.2021104
Figure
Reference
-
1. O'Hare T, Shakespeare WC, Zhu X, et al. 2009; AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 16:401–12. DOI: 10.1016/j.ccr.2009.09.028. PMID: 19878872. PMCID: PMC2804470.2. Nickel RS, Daves M, Keller F. 2015; Treatment of an adolescent with chronic myeloid leukemia and the T315I mutation with ponatinib. Pediatr Blood Cancer. 62:2050–1. DOI: 10.1002/pbc.25551. PMID: 25939962.
Article3. Yamamoto M, Hori T, Igarashi K, Shimada H, Tsutsumi H. 2018; Response to ponatinib before hematopoietic stem cell transplantation in a child with relapsed Philadelphia chromosome-positive acute lymphoblastic leukemia. Pediatr Int. 60:85–7. DOI: 10.1111/ped.13437. PMID: 29356285.
Article4. Gruner SE, Sayeed H, Suarez-Ferguson L, et al. 2019; Ponatinib use in two pediatric patients with relapsed Ph + ALL with ABL1 kinase domain mutations. Pediatr Hematol Oncol. 36:514–9. DOI: 10.1080/08880018.2019.1656685. PMID: 31510844.5. Aksu T, Ünal Ş, Gümrük F. 2020; The remarkable response to ponatinib therapy in a child with blastic phase of chronic myeloid leukemia. Turk J Pediatr. 62:479–81. DOI: 10.24953/turkjped.2020.03.016. PMID: 32558423.
Article6. Rossoff J, Huynh V, Rau RE, et al. 2020; Experience with ponatinib in paediatric patients with leukaemia. Br J Haematol. 189:363–8. DOI: 10.1111/bjh.16338. PMID: 31975387.
Article7. Millot F, Suttorp M, Versluys AB, et al. 2020; Ponatinib in childhood Philadelphia chromosome-positive leukaemias: an international registry of childhood chronic myeloid leukaemia study. Eur J Cancer. 136:107–12. DOI: 10.1016/j.ejca.2020.05.020. PMID: 32668374.
Article8. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. 2018; Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 132:393–404. DOI: 10.1182/blood-2016-09-739086. PMID: 29567798. PMCID: PMC6071555.
Article9. Le Coutre PD, Kim DW, Pinilla-Ibarz J, et al. 2013; Ponatinib in heavily pretreated patients with chronic phase chronic myeloid leukemia (CPCML): management of adverse events (AEs). Blood (ASH Annual Meeting Abstract). 122(Suppl):abst 1496. DOI: 10.1182/blood.V122.21.1496.1496.
Article10. Dorer DJ, Knickerbocker RK, Baccarani M, et al. 2016; Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 48:84–91. DOI: 10.1016/j.leukres.2016.07.007. PMID: 27505637.
Article11. DeFilipp Z, Langston AA, Chen Z, et al. 2016; Does post-transplant maintenance therapy with tyrosine kinase inhibitors improve outcomes of patients with high-risk Philadelphia chromosome-positive leukemia? Clin Lymphoma Myeloma Leuk. 16:466–71. DOI: 10.1016/j.clml.2016.04.017. PMID: 27297665.
Article12. Sasaki H, Mitani S, Kusumoto S, et al. 2019; Pre- and post-transplant ponatinib for a patient with acute megakaryoblastic blast phase chronic myeloid leukemia with T315I mutation who underwent allogeneic hematopoietic stem cell transplantation. Int J Hematol. 110:119–23. DOI: 10.1007/s12185-019-02628-8. PMID: 30879266.
Article13. Hess G, Bunjes D, Siegert W, et al. 2005; Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol. 23:7583–93. DOI: 10.1200/JCO.2005.01.3110. PMID: 16234522.
Article14. Mahon FX, Réa D, Guilhot J, et al. 2010; Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 11:1029–35. DOI: 10.1016/S1470-2045(10)70233-3. PMID: 20965785.
Article15. de Bruijn CMA, Millot F, Suttorp M, et al. 2019; Discontinuation of imatinib in children with chronic myeloid leukaemia in sustained deep molecular remission: results of the STOP IMAPED study. Br J Haematol. 185:718–24. DOI: 10.1111/bjh.15826. PMID: 30843196.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment after failure of frontline therapy of chronic myeloid leukemia in chronic phase including allogeneic hematopoietic stem cell transplantation
- Chronic graft versus host disease with small bowel obstruction after unrelated hematopoietic stem cell transplantation in a patient with acute myeloid leukemia
- A Case Report of the Second de Novo Acute Myeloid Leukemia (AML) Following Allogeneic Stem Cell Transplantation in a Patient with the First AML
- Chronic Myeloid Leukemia Relapsing as Isolated Extramedullary Disease Presenting as a Cardiac Mass
- Two Cases of Chronic Myeloid Leukemia in Lymphoid Blast Phase Presented as Philadelphia-Positive Acute Lymphoblastic Leukemia