Blood Res.
2021 Dec;56(4):322-331. 10.5045/br.2021.2021101.
Real-life ruxolitinib experience in intermediate-risk myelofibrosis
- Affiliations
-
- 1Department of Hematology, Marmara University Hospital, Istanbul, Turkey
- KMID: 2524092
- DOI: http://doi.org/10.5045/br.2021.2021101
Abstract
- Background
In this retrospective cohort of patients with primary, post-polycythemia vera, or post-essential thrombocythemia myelofibrosis, 57 patients with MF who received ruxolitinib for MF-related symptoms or symptomatic splenomegaly were evaluated.
Methods
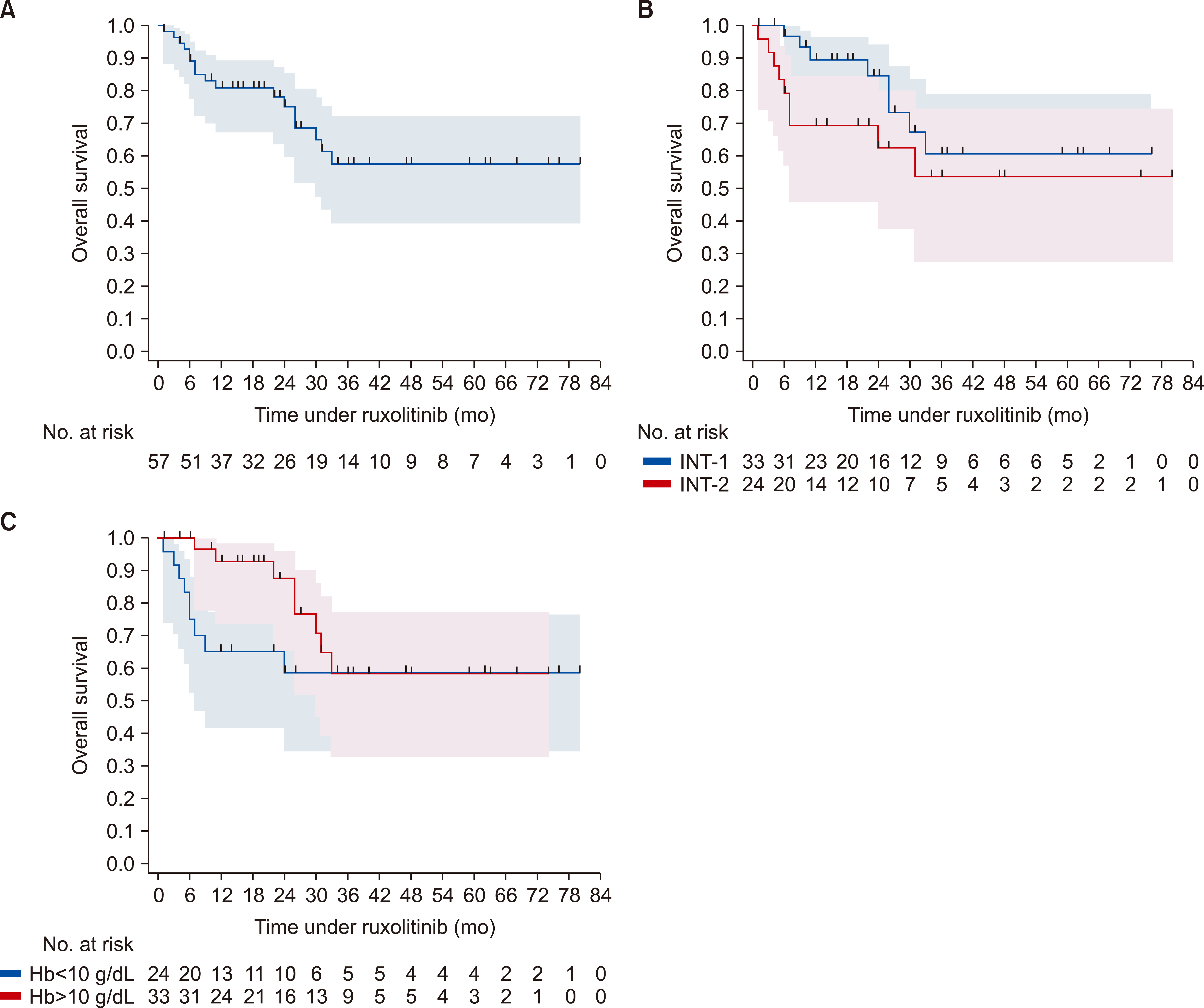
The median age of the patients in this cohort was approximately 58 years. Of these, there were 33 patients (57.9%) in INT-1, 23 patients (40.4%) in INT-2, and 1 patient (1.8%) at high risk. Overall, spleen size reduction of at least 35% (spleen response) was achieved in 56.6% and 63.3% of all cohort and INT-1 risk at any time, respectively.
Results
Symptom response and clinical improvement were observed in 21.7% and 60.7% of patients, respectively. Anemia and thrombocytopenia were prevalent, but manageable. About 73.7% of patients continued treatment during a median follow-up of 22 months. Two-year OS probability was approximately 84.5% (95% CI, 63.1‒94.0%) and 62.3% (95% CI, 37.5‒79.6%) for the intermediate-1 and -2 risk groups, respectively.
Conclusion
Real-life experience in a community-based hospital confirms the efficacy and safety profile of ruxolitinib in intermediate-risk myelofibrosis. Treatment discontinuation rates were lower than those in clinical trials.
Keyword
Figure
Reference
-
1. Cervantes F. 2014; How I treat myelofibrosis. Blood. 124:2635–42. DOI: 10.1182/blood-2014-07-575373. PMID: 25232060.
Article2. Passamonti F, Cervantes F, Vannucchi AM, et al. 2010; A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 115:1703–8. DOI: 10.1182/blood-2009-09-245837. PMID: 20008785.
Article3. Gangat N, Caramazza D, Vaidya R, et al. 2011; DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 29:392–7. DOI: 10.1200/JCO.2010.32.2446. PMID: 21149668.
Article4. Guglielmelli P, Lasho TL, Rotunno G, et al. 2018; MIPSS70: Mutation-Enhanced International Prognostic Score System for trans-plantation-age patients with primary myelofibrosis. J Clin Oncol. 36:310–8. DOI: 10.1200/JCO.2017.76.4886. PMID: 29226763.
Article5. Tefferi A, Guglielmelli P, Lasho TL, et al. 2018; MIPSS70+ Version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for primary myelofibrosis. J Clin Oncol. 36:1769–70. DOI: 10.1200/JCO.2018.78.9867. PMID: 29708808.
Article6. Tefferi A, Guglielmelli P, Nicolosi M, et al. 2018; GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia. 32:1631–42. DOI: 10.1038/s41375-018-0107-z. PMID: 29654267. PMCID: PMC6035151.
Article7. Scott BL, Gooley TA, Sorror ML, et al. 2012; The Dynamic International Prognostic Scoring System for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood. 119:2657–64. DOI: 10.1182/blood-2011-08-372904. PMID: 22234678. PMCID: PMC3311279.
Article8. Harrison C, Kiladjian JJ, Al-Ali HK, et al. 2012; JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 366:787–98. DOI: 10.1056/NEJMoa1110556. PMID: 22375970.
Article9. Verstovsek S, Mesa RA, Gotlib J, et al. 2012; A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 366:799–807. DOI: 10.1056/NEJMoa1110557. PMID: 22375971. PMCID: PMC4822164.
Article10. Al-Ali HK, Griesshammer M, le Coutre P, et al. 2016; Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica. 101:1065–73. DOI: 10.3324/haematol.2016.143677. PMID: 27247324. PMCID: PMC5060023.
Article11. Arber DA, Orazi A, Hasserjian R, et al. 2016; The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127:2391–405. DOI: 10.1182/blood-2016-03-643544. PMID: 27069254.
Article12. Cervantes F, Dupriez B, Pereira A, et al. 2009; New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 113:2895–901. DOI: 10.1182/blood-2008-07-170449. PMID: 18988864.
Article13. Gupta V, Harrison C, Hexner EO, et al. 2016; The impact of anemia on overall survival in patients with myelofibrosis treated with ruxolitinib in the COMFORT studies. Haematologica. 101:e482–4. DOI: 10.3324/haematol.2016.151449. PMID: 27587385. PMCID: PMC5479619.
Article14. Tefferi A, Cervantes F, Mesa R, et al. 2013; Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 122:1395–8. DOI: 10.1182/blood-2013-03-488098. PMID: 23838352. PMCID: PMC4828070.
Article15. Mead AJ, Milojkovic D, Knapper S, et al. 2015; Response to ruxolitinib in patients with intermediate-1-, intermediate-2-, and high-risk myelofibrosis: results of the UK ROBUST Trial. Br J Haematol. 170:29–39. DOI: 10.1111/bjh.13379. PMID: 25824940.
Article16. Palandri F, Tiribelli M, Benevolo G, et al. 2018; Efficacy and safety of ruxolitinib in intermediate-1 IPSS risk myelofibrosis patients: results from an independent study. Hematol Oncol. 36:285–90. DOI: 10.1002/hon.2429. PMID: 28512865.
Article17. Harrison CN, Vannucchi AM, Kiladjian JJ, et al. 2016; Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 30:1701–7. DOI: 10.1038/leu.2016.148. PMID: 27211272. PMCID: PMC5399157.
Article18. Heine A, Held SA, Daecke SN, et al. 2013; The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 122:1192–202. DOI: 10.1182/blood-2013-03-484642. PMID: 23770777.
Article19. Hong J, Lee JH, Byun JM, et al. 2019; Risk of disease transformation and second primary solid tumors in patients with myeloproliferative neoplasms. Blood Adv. 3:3700–8. DOI: 10.1182/bloodadvances.2019000655. PMID: 31765478. PMCID: PMC6880910.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ruxolitinib changes the natural course of myelofibrosis and its transplant outcome
- Evolution of Myelofibrosis Treatment
- EBV reactivation mimicking a lymphoproliferative disorder associated with ruxolitinib therapy for myelofibrosis
- Rapidly Growing and Aggressive Cutaneous Squamous Cell Carcinomas in a Patient Treated with Ruxolitinib
- Coexistence of BCR/ABL1-positive chronic myeloid leukemia and JAK2 V617F-mutated myelofibrosis successfully treated with dasatinib and ruxolitinib