Ann Dermatol.
2019 Apr;31(2):204-208. 10.5021/ad.2019.31.2.204.
Rapidly Growing and Aggressive Cutaneous Squamous Cell Carcinomas in a Patient Treated with Ruxolitinib
- Affiliations
-
- 1Department of Dermatology, Hospital del Mar, Parc de Salut Mar, Barcelona, Spain. 61693@parcdesalutmar.cat
- 2Department of Pathology, Hospital del Mar, Parc de Salut Mar, Barcelona, Spain.
- 3Department of Hematology, Hospital del Mar, Parc de Salut Mar, Barcelona, Spain.
- KMID: 2439067
- DOI: http://doi.org/10.5021/ad.2019.31.2.204
Abstract
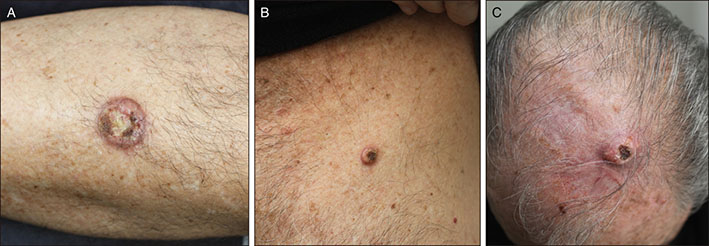
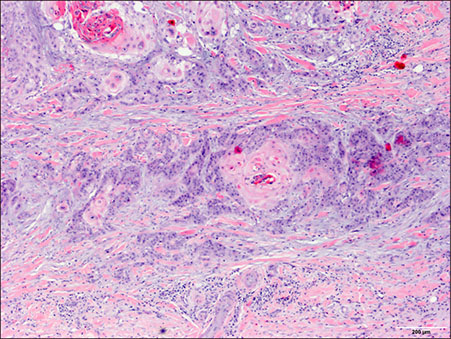
- Ruxolitinib is a Janus kinase (JAK)1 and JAK2 inhibitor approved for the treatment of myelofibrosis and for polycythemia patients who are resistant or intolerant to hydroxyurea. We report a 72 year-old man patient with polycythemia vera who developed multiple cutaneous squamous cell carcinomas (cSCCs) with keratoacanthoma-like histological features while on treatment with ruxolitinib. Similar lesions have been reported in an isolated patient who also received ruxolitinib. Our case confirms that ruxolitinib may induce eruptive cSCCs with characteristic clinical and histological features that differentiate them from conventional non-drug induced lesions. Moreover, we performed a mutational panel analysis of the tumors. The lack of specific mutations in these tumors suggests an impairment of immunosurveillance in the origin of the cutaneous lesions. Frequent and thorough dermatological examinations in patients receiving ruxolitinib with a history of photodamage, skin cancer and/or previous hydroxyurea intake is thus recommended.
Keyword
MeSH Terms
Figure
Reference
-
1. Rane SG, Reddy EP. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 2002; 21:3334–3358.
Article2. Jatiani SS, Baker SJ, Silverman LR, Reddy EP. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010; 1:979–993.
Article3. Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. U.S. Food and Drug Administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012; 18:3212–3217.
Article4. Caocci G, Murgia F, Podda L, Solinas A, Atzeni S, La Nasa G. Reactivation of hepatitis B virus infection following ruxolitinib treatment in a patient with myelofibrosis. Leukemia. 2014; 28:225–227.
Article5. Goldberg RA, Reichel E, Oshry LJ. Bilateral toxoplasmosis retinitis associated with ruxolitinib. N Engl J Med. 2013; 369:681–683.
Article6. Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012; 366:799–807.
Article7. Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015; 372:426–435.
Article8. Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015; 100:479–488.
Article9. Shreberk-Hassidim R, Ramot Y, Zlotogorski A. Janus kinase inhibitors in dermatology: a systematic review. J Am Acad Dermatol. 2017; 76:745–753.e19.
Article10. Fabiano A, Calzavara-Pinton P, Monari P, Moggio E, Pellacani G, Manganoni AM, et al. Eruptive squamous cell carcinomas with keratoacanthoma-like features in a patient treated with ruxolitinib. Br J Dermatol. 2015; 173:1098–1099.
Article11. Ogita A, Ansai SI, Misago N, Anan T, Fukumoto T, Saeki H. Histopathological diagnosis of epithelial crateriform tumors: keratoacanthoma and other epithelial crateriform tumors. J Dermatol. 2016; 43:1321–1331.
Article12. Smith KJ, Haley H, Hamza S, Skelton HG. Eruptive keratoacanthoma-type squamous cell carcinomas in patients taking sorafenib for the treatment of solid tumors. Dermatol Surg. 2009; 35:1766–1770.
Article13. Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012; 30:316–321.
Article14. Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015; 43:D805–D811.
Article15. Parampalli Yajnanarayana S, Stübig T, Cornez I, Alchalby H, Schönberg K, Rudolph J, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol. 2015; 169:824–833.
Article16. Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014; 123:3832–3842.
Article17. Kosmidis M, Dziunycz P, Suárez-Fariñas M, Mühleisen B, Schärer L, Läuchli S, et al. Immunosuppression affects CD4+ mRNA expression and induces Th2 dominance in the microenvironment of cutaneous squamous cell carcinoma in organ transplant recipients. J Immunother. 2010; 33:538–546.
Article18. Harwood CA, Toland AE, Proby CM, Euvrard S, Hofbauer GFL, Tommasino M, et al. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017; 177:1217–1224.
Article19. Sanchez-Palacios C, Guitart J. Hydroxyurea-associated squamous dysplasia. J Am Acad Dermatol. 2004; 51:293–300.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Squamous Cell Carcinomas Arising from chronic Osteomyelitic Sinuses: A Report of Three cases
- A Giant Keratoacanthoma Treated with Surgical Excision
- Immunohistochemical Identification of Keratin in Cutaneous Tumors
- Xeroderma Pigmentosum: The Treatment of Associated Skin Cancer
- Bone-Invaded Squamous Cell Carcinoma with Aggressive Behavior