Korean Circ J.
2021 Dec;51(12):1017-1029. 10.4070/kcj.2021.0076.
Association between the Use of Diuretics and Size Reduction in Pediatric Atrial Septal Defect
- Affiliations
-
- 1Department of Pediatrics, Korea University College of Medicine and Korea University Medical Center, Seoul, Korea
- 2Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Children's Hospital, Seoul, Korea
- 3Seoul National University College of Medicine, Seoul, Korea
- KMID: 2523154
- DOI: http://doi.org/10.4070/kcj.2021.0076
Abstract
- Background and Objectives
While diuretics are sometimes used in atrial septal defect (ASD) treatment, their effect on ASD size reduction remains unclear. We aimed to evaluate the efficacy of diuretics in ASD size reduction in pediatric patients.
Methods
We retrospectively reviewed the medical records of patients with secundum ASD (size ≥10 mm), between 2005 and 2019. Patients were divided into two groups based on the diuretic administration.
Results
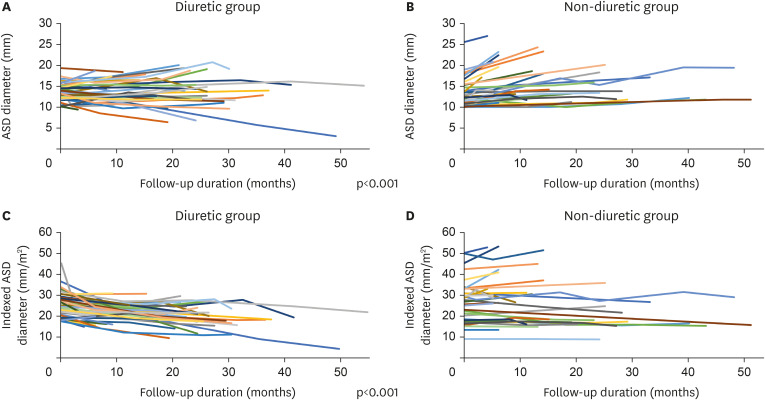
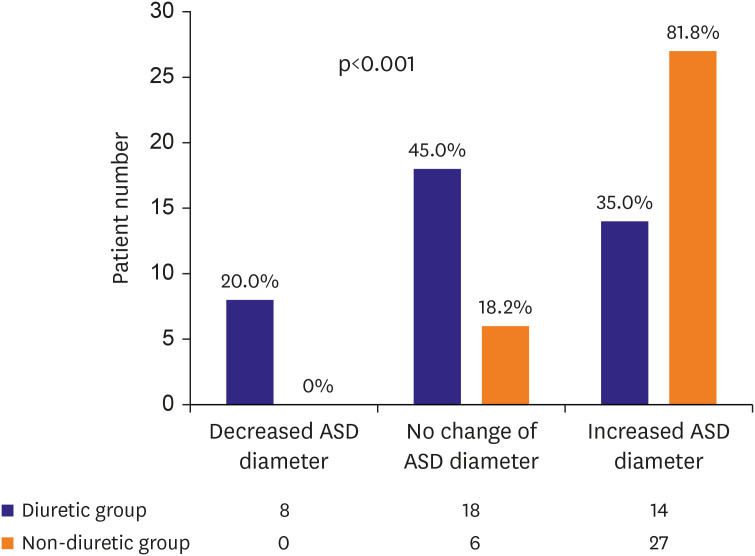
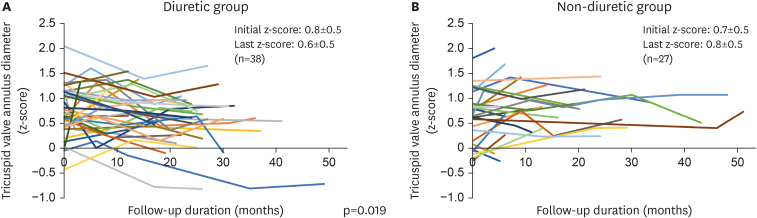
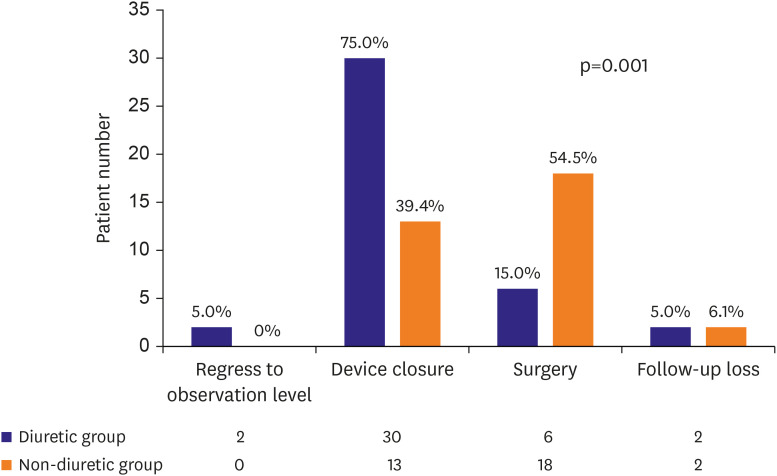
Of the 73 enrolled patients, 40 received diuretics. The initial age at ASD diagnosis (2.8±1.7 vs. 2.5±2.0 years, p=0.526) and follow-up duration (22.3±11.4 vs. 18.7±13.2 months, p=0.224) were not significantly different between the groups. The ASD diameter at the initial diagnosis (13.7±2.0 vs. 13.5±3.4 mm, p=0.761) and the indexed ASD diameter (25.5±5.9 vs. 26.9±10.3 mm/m2 , p=0.493) were also not significantly different between two groups. The ASD diameter significantly increased in the non-diuretic group during follow-up (0.0±2.9 vs. +2.6±2.0 mm, p<0.001). The indexed ASD diameter significantly decreased in the diuretic group during follow-up (−5.7±6.5 vs. +0.2±3.9 mm/m 2 , p<0.001). In the linear mixed model analysis, diuretic use was associated with ASD diameter decrease (p<0.001) and indexed ASD diameter reduction (p<0.001) over time. Device closure was more frequently performed in the diuretic (75.0%) than in the non-diuretic group (39.4%).
Conclusions
Patients receiving diuretics are less likely to undergo surgery. The diuretics administration may be associated with the use of smaller ASD devices for transcatheter treatment through ASD size reduction.
Keyword
Figure
Reference
-
1. Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss & Adams' heart disease in infants, children, and adolescents: including the fetus and young adult. Philadelphia (PA): Lippincott Williams & Wilkins;2013.2. Baumgartner H, De Backer J, Babu-Narayan SV, et al. ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2020; 41:4153–4154. PMID: 33128054.3. Nyboe C, Olsen MS, Nielsen-Kudsk JE, Hjortdal VE. Atrial fibrillation and stroke in adult patients with atrial septal defect and the long-term effect of closure. Heart. 2015; 101:706–711. PMID: 25691512.
Article4. Ooi YK, Kelleman M, Ehrlich A, et al. Transcatheter versus surgical closure of atrial septal defects in children: a value comparison. JACC Cardiovasc Interv. 2016; 9:79–86. PMID: 26762915.5. Butera G, Biondi-Zoccai G, Sangiorgi G, et al. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention. 2011; 7:377–385. PMID: 21729841.
Article6. Rao PS, Harris AD. Recent advances in managing septal defects: atrial septal defects. F1000 Res. 2017; 6:2042.
Article7. Kim JH, Yun S, Hwang SS, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–149. PMID: 29853938.
Article8. Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008; 21:922–934. PMID: 18406572.
Article9. Losay J, Petit J, Lambert V, et al. Percutaneous closure with Amplatzer device is a safe and efficient alternative to surgery in adults with large atrial septal defects. Am Heart J. 2001; 142:544–548. PMID: 11526371.
Article10. Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005; 45:505–507. PMID: 15708695.
Article11. Jalal Z, Hascoët S, Gronier C, et al. Long-term outcomes after percutaneous closure of ostium secundum atrial septal defect in the young: a nationwide cohort study. JACC Cardiovasc Interv. 2018; 11:795–804. PMID: 29673513.12. Jung SY, Kim AY, Jung JW, Choi JY. Procedural, early and long-term outcomes after percutaneous closure of atrial septal defect: comparison between large and very large atrial septal defect groups. Korean Circ J. 2019; 49:975–986. PMID: 31165594.
Article13. De Wolf D. Complications of transcatheter atrial septal defect closure. Interv Cardiol (Lond). 2009; 1:209–218.
Article14. Petit CJ, Justino H, Pignatelli RH, Crystal MA, Payne WA, Ing FF. Percutaneous atrial septal defect closure in infants and toddlers: predictors of success. Pediatr Cardiol. 2013; 34:220–225. PMID: 22806712.
Article15. Saritas T, Yucel IK, Demir IH, Demir F, Erdem A, Celebi A. Comparison of transcatheter atrial septal defect closure in children, adolescents and adults: differences, challenges and short-, mid- and long-term results. Korean Circ J. 2016; 46:851–861. PMID: 27826346.
Article16. Butera G, De Rosa G, Chessa M, et al. Transcatheter closure of atrial septal defect in young children: results and follow-up. J Am Coll Cardiol. 2003; 42:241–245. PMID: 12875758.
Article17. Bartakian S, Fagan TE, Schaffer MS, Darst JR. Device closure of secundum atrial septal defects in children <15 kg: complication rates and indications for referral. JACC Cardiovasc Interv. 2012; 5:1178–1184. PMID: 23174643.18. Tanghöj G, Odermarsky M, Naumburg E, Liuba P. Early complications after percutaneous closure of atrial septal defect in infants with procedural weight less than 15 kg. Pediatr Cardiol. 2017; 38:255–263. PMID: 27837301.
Article19. Brassard M, Fouron JC, van Doesburg NH, Mercier LA, De Guise P. Outcome of children with atrial septal defect considered too small for surgical closure. Am J Cardiol. 1999; 83:1552–1555. PMID: 10363870.
Article20. Hsu DT, Pearson GD. Heart failure in children: part II: diagnosis, treatment, and future directions. Circ Heart Fail. 2009; 2:490–498. PMID: 19808380.21. Ding D, Liu H, Qi W, et al. Ototoxic effects and mechanisms of loop diuretics. J Otol. 2016; 11:145–156. PMID: 29937824.
Article22. Lin BM, Curhan SG, Wang M, Eavey R, Stankovic KM, Curhan GC. Hypertension, diuretic use, and risk of hearing loss. Am J Med. 2016; 129:416–422. PMID: 26656761.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Atrial Septal Defect in Identical Twins
- Transcatheter Closure of Secundum Atrial Septal Defect with the Amplatzer Septal Occluder
- Transcatheter Closure of Atrial Septal Defect with CardioSEAL(R) and STARFlex(R) Septal Occlusion Devices
- The Natural Course and Size Change of Atrial Septal Defect Secundum
- Comparison of Transcatheter Atrial Septal Defect Closure in Children, Adolescents and adults: Differences, Challenges and Short-, Mid- and Long-Term Results