Ann Surg Treat Res.
2021 Dec;101(6):315-321. 10.4174/astr.2021.101.6.315.
Analysis of risk factors associated with survival in human epidermal growth factor receptor 2-positive ductal carcinoma in situ using Korean Breast Cancer Society Database
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Ewha Womans University Cancer Center Hospital for Women, Seoul, Korea
- 3Cancer for Breast Cancer, National Cancer Center, Goyang, Korea
- 4Department of Surgery, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea
- 5Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Surgery, Jeonbuk National University College of Medicine, Jeonju, Korea
- KMID: 2522927
- DOI: http://doi.org/10.4174/astr.2021.101.6.315
Abstract
- Purpose
This study was performed to identify the risk of mortality in patients diagnosed with human epidermal growth factor receptor 2 (HER2)-positive ductal carcinoma in situ (DCIS).
Methods
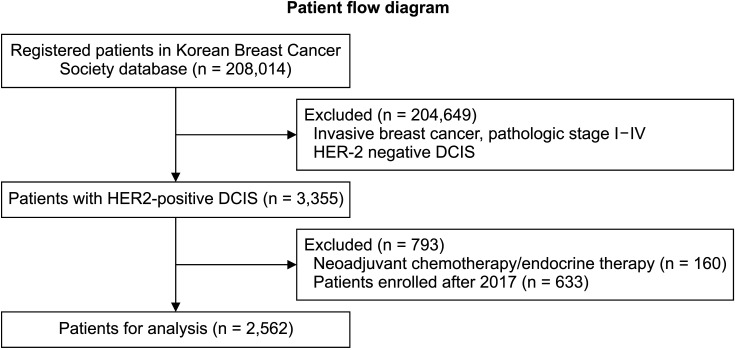
We selected 2,592 patients with HER2-positive DCIS from Korean Breast Cancer Society (KBCS) database between January 1997 and December 2019. Patients who received neoadjuvant chemotherapy were excluded. Logistic regression analysis was used to determine the association between clinical factors and overall death after adjusting for tumor and clinical characteristics. Mortality data were modified using the Statistics Korea data.
Results
Thirty deaths (1.2%) were identified out of 2,592 patients in the KBCS database. In the univariate logistic regression analysis, older age, higher body mass index (BMI), type of breast surgery (mastectomy), estrogen receptornegative, progesterone receptor-negative, and exposure to endocrine therapy were significant clinical factors associated with death. In the multivariate analysis, age (hazard ratio [HR], 1.062; 95% confidence interval [CI], 1.015–1.111; P = 0.006), BMI (HR, 1.179; 95% CI, 1.032–1.347, P = 0.016), breast surgery type (mastectomy vs. lumpectomy; HR, 0.285; 95% CI, 0.096–0.844; P = 0.024), and endocrine therapy (HR, 0.314; 95% CI, 0.099–0.995; P = 0.049) were significant risk factors for mortality.
Conclusion
Advanced age, higher BMI, mastectomy, and the absence of endocrine therapy were factors associated with poor survival of patients with HER2-positive DCIS. This finding requires further validation combined with additional analysis of large databases.
Keyword
Figure
Reference
-
1. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer Statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020; 52:335–350. PMID: 32178489.
Article2. Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982; 49:751–758. PMID: 6275978.
Article3. Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006; 19:617–621. PMID: 16528377.
Article4. Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012; 30:1268–1273. PMID: 22393101.
Article5. Hoque A, Sneige N, Sahin AA, Menter DG, Bacus JW, Hortobagyi GN, et al. Her-2/neu gene amplification in ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2002; 11:587–590. PMID: 12050101.6. Curigliano G, Disalvatore D, Esposito A, Pruneri G, Lazzeroni M, Guerrieri-Gonzaga A, et al. Risk of subsequent in situ and invasive breast cancer in human epidermal growth factor receptor 2-positive ductal carcinoma in situ. Ann Oncol. 2015; 26:682–687. PMID: 25600567.7. Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998; 16:441–452. PMID: 9469327.
Article8. Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999; 353:1993–2000. PMID: 10376613.
Article9. Claus EB, Chu P, Howe CL, Davison TL, Stern DF, Carter D, et al. Pathobiologic findings in DCIS of the breast: morphologic features, angiogenesis, HER-2/neu and hormone receptors. Exp Mol Pathol. 2001; 70:303–316. PMID: 11418009.
Article10. Collins LC, Schnitt SJ. HER2 protein over expression in estrogen receptor-positive ductal carcinoma in situ of the breast: frequency and implications for tamoxifen therapy. Mod Pathol. 2005; 18:615–620. PMID: 15696127.11. Cornfield DB, Palazzo JP, Schwartz GF, Goonewardene SA, Kovatich AJ, Chervoneva I, et al. The prognostic significance of multiple morphologic features and biologic markers in ductal carcinoma in situ of the breast: a study of a large cohort of patients treated with surgery alone. Cancer. 2004; 100:2317–2327. PMID: 15160334.12. Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998; 16:413–428. PMID: 9831867.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Epidermal Growth Factor Receptor 2-Subtype Invasive Ductal Carcinoma Recurring as Basal-Human Epidermal Growth Factor Receptor 2-Subtype Squamous Cell Carcinoma
- EGFR Gene Amplification and Protein Expression in Invasive Ductal Carcinoma of the Breast
- Prognostic Implications of MicroRNA-21 Overexpression in Invasive Ductal Carcinomas of the Breast
- Clinicopathological Significance of SMAD4 Expression in Breast Cancer
- Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy