Nutr Res Pract.
2021 Dec;15(6):686-702. 10.4162/nrp.2021.15.6.686.
Schisandrae Fructus ethanol extract attenuates particulate matter 2.5-induced inflammatory and oxidative responses by blocking the activation of the ROS-dependent NFκB signaling pathway
- Affiliations
-
- 1Anti-Aging Research Center, Dong-Eui University, Busan 47340, Korea
- 2Department of Biochemistry, Dong-Eui University College of Korean Medicine, Busan 47227, Korea
- 3Division of Basic Sciences, College of Liberal Studies, Dong-Eui University, Busan 47340, Korea
- 4Nakdonggang National Institute of Biological Resources, Sangju 37242, Korea
- 5Department of Marine Life Sciences, Jeju National University, Jeju 63243, Korea
- 6Department of Food and Nutrition, Dong-Eui University, Busan 47340, Korea
- KMID: 2522650
- DOI: http://doi.org/10.4162/nrp.2021.15.6.686
Abstract
- BACKGROUND/OBJECTIVES
Schisandrae Fructus, the fruit of Schisandra chinensis Baill., has traditionally been used as a medicinal herb for the treatment of various diseases, and has proven its various pharmacological effects, including anti-inflammatory and antioxidant activities. In this study, we investigated the inhibitory effect of Schisandrae Fructus ethanol extract (SF) on inflammatory and oxidative stress in particulate matter 2.5 (PM2.5)-treated RAW 264.7 macrophages.
MATERIALS/METHODS
To investigate the anti-inflammatory and antioxidant effects of SF in PM2.5-stimulated RAW 264.7 cells, the levels of pro-inflammatory mediator such as nitric oxide (NO) and prostaglandin E2 (PGE2 ), cytokines including interleukin (IL)-6 and IL-1β, and reactive oxygen species (ROS) were measured. To elucidate the mechanism underlying the effect of SF, the expression of genes involved in the generation of inflammatory factors was also investigated. We further evaluated the anti-inflammatory and antioxidant efficacy of SF against PM2.5 in the zebrafish model.
RESULTS
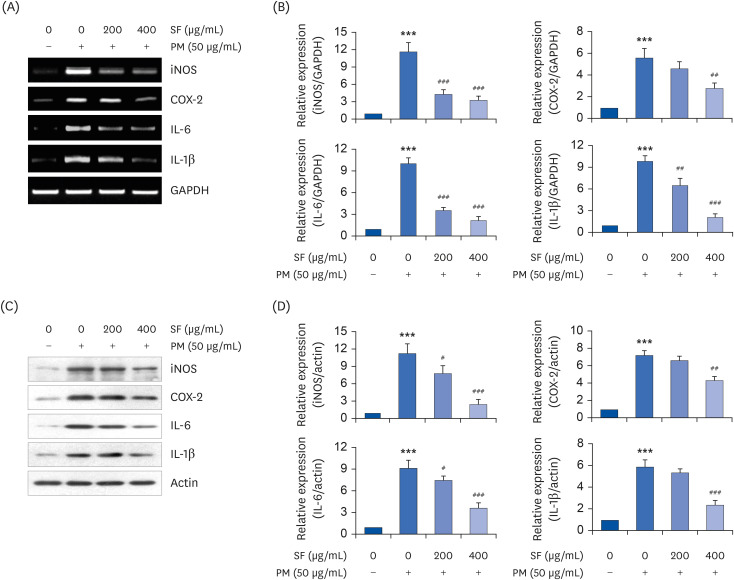
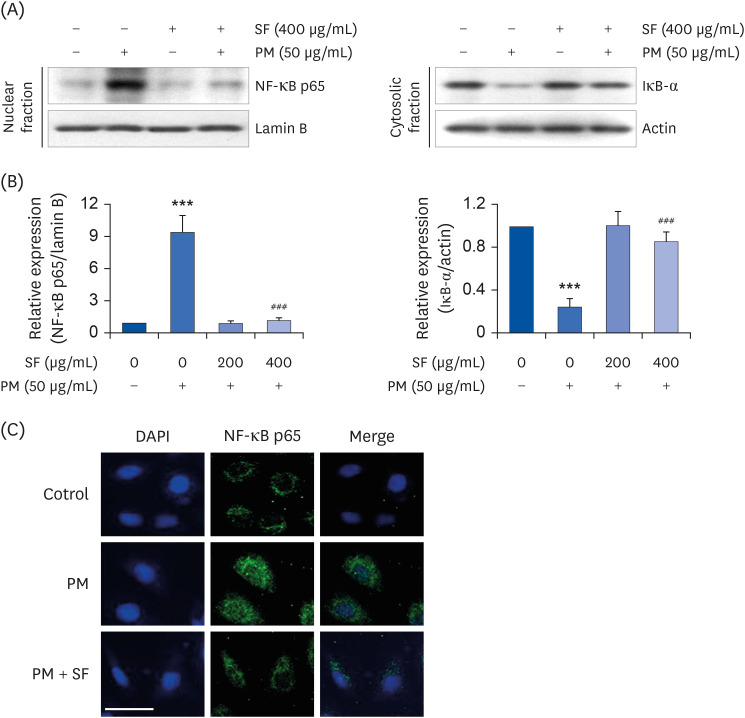
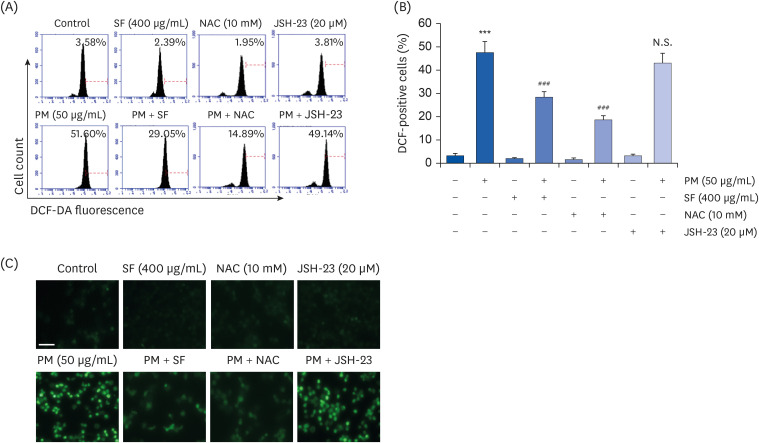
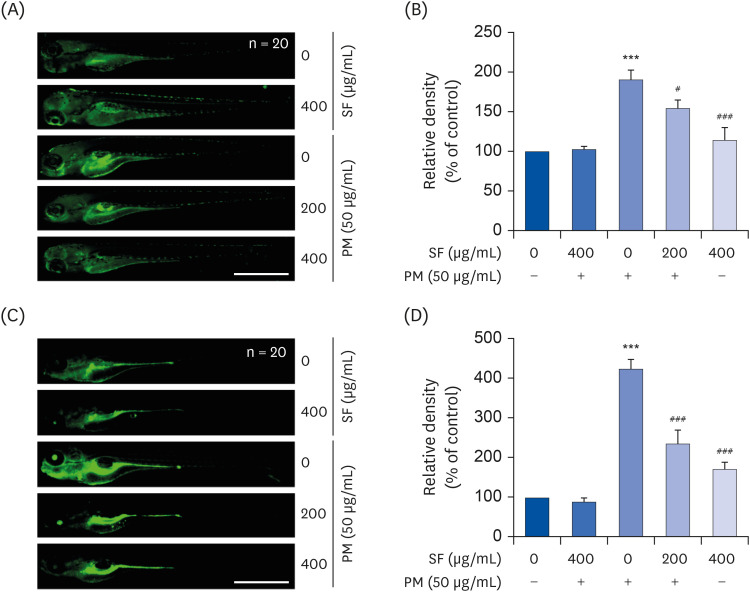
The results indicated that SF treatment significantly inhibited the PM2.5-induced release of NO and PGE2 , which was associated with decreased inducible NO synthase and cyclooxygenase-2 expression. SF also attenuated the PM2.5-induced expression of IL-6 and IL-1β, reducing their extracellular secretion. Moreover, SF suppressed the PM2.5-mediated translocation of nuclear factor-kappa B (NF-κB) from the cytosol into nuclei and the degradation of inhibitor IκB-α, indicating that SF exhibited anti-inflammatory effects by inhibiting the NF-κB signaling pathway. In addition, SF abolished PM2.5-induced generation of ROS, similar to the pretreatment of a ROS scavenger, but not by an inhibitor of NF-κB activity. Furthermore, SF showed strong protective effects against NO and ROS production in PM2.5-treated zebrafish larvae.
CONCLUSIONS
Our findings suggest that SF exerts anti-inflammatory and antioxidant effects against PM2.5 through ROS-dependent down-regulating the NF-κB signaling pathway, and that SF can be a potential functional substance to prevent PM2.5-mediated inflammatory and oxidative damage.
Figure
Cited by 1 articles
-
L -Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
Zhengxuan Wang, Mingcai Liang, Hui Li, Bingxiao Liu, Lin Yang
Nutr Res Pract. 2022;16(6):729-744. doi: 10.4162/nrp.2022.16.6.729.
Reference
-
1. Loxham M, Nieuwenhuijsen MJ. Health effects of particulate matter air pollution in underground railway systems - a critical review of the evidence. Part Fibre Toxicol. 2019; 16:12. PMID: 30841934.
Article2. Almetwally AA, Bin-Jumah M, Allam AA. Ambient air pollution and its influence on human health and welfare: an overview. Environ Sci Pollut Res Int. 2020; 27:24815–24830. PMID: 32363462.
Article3. Lynch HN, Loftus CT, Cohen JM, Kerper LE, Kennedy EM, Goodman JE. Weight-of-evidence evaluation of associations between particulate matter exposure and biomarkers of lung cancer. Regul Toxicol Pharmacol. 2016; 82:53–93. PMID: 27765718.
Article4. Wu JZ, Ge DD, Zhou LF, Hou LY, Zhou Y, Li QY. Effects of particulate matter on allergic respiratory diseases. Chronic Dis Transl Med. 2018; 4:95–102. PMID: 29988900.
Article5. Combes A, Franchineau G. Fine particle environmental pollution and cardiovascular diseases. Metabolism. 2019; 100S:153944. PMID: 31610849.
Article6. Liu B, Wu J, Zhang J, Wang L, Yang J, Liang D, Dai Q, Bi X, Feng Y, Zhang Y, Zhang Q. Characterization and source apportionment of PM2.5 based on error estimation from EPA PMF 5.0 model at a medium city in China. Environ Pollut. 2017; 222:10–22. PMID: 28088626.7. Yang Y, Ruan Z, Wang X, Yang Y, Mason TG, Lin H, Tian L. Short-term and long-term exposures to fine particulate matter constituents and health: a systematic review and meta-analysis. Environ Pollut. 2019; 247:874–882. PMID: 30731313.
Article8. He M, Ichinose T, Yoshida S, Ito T, He C, Yoshida Y, Arashidani K, Takano H, Sun G, Shibamoto T. PM2.5-induced lung inflammation in mice: differences of inflammatory response in macrophages and type II alveolar cells. J Appl Toxicol. 2017; 37:1203–1218. PMID: 28555929.
Article9. Tang Q, Huang K, Liu J, Wu S, Shen D, Dai P, Li C. Fine particulate matter from pig house induced immune response by activating TLR4/MAPK/NF-κB pathway and NLRP3 inflammasome in alveolar macrophages. Chemosphere. 2019; 236:124373. PMID: 31336238.
Article10. Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, Vacek P, Mossman BT. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro . Am J Respir Cell Mol Biol. 2000; 23:182–187. PMID: 10919984.11. Jeong S, Park SA, Park I, Kim P, Cho NH, Hyun JW, Hyun YM. PM2.5 Exposure in the respiratory system induces distinct inflammatory signaling in the lung and the liver of mice. J Immunol Res. 2019; 2019:3486841. PMID: 31871955.
Article12. Wyatt LH, Devlin RB, Rappold AG, Case MW, Diaz-Sanchez D. Low levels of fine particulate matter increase vascular damage and reduce pulmonary function in young healthy adults. Part Fibre Toxicol. 2020; 17:58. PMID: 33198760.
Article13. Piao MJ, Ahn MJ, Kang KA, Ryu YS, Hyun YJ, Shilnikova K, Zhen AX, Jeong JW, Choi YH, Kang HK, Koh YS, Hyun JW. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch Toxicol. 2018; 92:2077–2091. PMID: 29582092.
Article14. Lee H, Hwang-Bo H, Ji SY, Kim MY, Kim SY, Park C, Hong SH, Kim GY, Song KS, Hyun JW, Choi YH. Diesel particulate matter2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species. Environ Pollut. 2020; 262:114301. PMID: 32155554.
Article15. Wei H, Yuan W, Yu H, Geng H. Cytotoxicity induced by fine particulate matter (PM2.5) via mitochondria-mediated apoptosis pathway in rat alveolar macrophages. Environ Sci Pollut Res Int. 2021; 21:1–11.16. Rao X, Zhong J, Brook RD, Rajagopalan S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid Redox Signal. 2018; 28:797–818. PMID: 29084451.
Article17. Øvrevik J. Oxidative potential versus biological effects: a review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int J Mol Sci. 2019; 20:4772.
Article18. Guan L, Geng X, Stone C, Cosky EE, Ji Y, Du H, Zhang K, Sun Q, Ding Y. PM2.5 exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ Toxicol. 2019; 34:530–538. PMID: 30672636.19. Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008; 118:183–212. PMID: 18515024.20. Zhang M, Xu L, Yang H. Schisandra chinensis Fructus and its active ingredients as promising resources for the treatment of neurological diseases. Int J Mol Sci. 2018; 19:1970.21. Chun JN, Cho M, So I, Jeon JH. The protective effects of Schisandra chinensis fruit extract and its lignans against cardiovascular disease: a review of the molecular mechanisms. Fitoterapia. 2014; 97:224–233. PMID: 24976588.22. Nowak A, Zakłos-Szyda M, Błasiak J, Nowak A, Zhang Z, Zhang B. Potential of Schisandra chinensis (Turcz.) Baill. in human health and nutrition: a review of current knowledge and therapeutic perspectives. Nutrients. 2019; 11:333.23. Song FJ, Zeng KW, Chen JF, Li Y, Song XM, Tu PF, Wang XM. Extract of Fructus Schisandrae chinensis inhibits neuroinflammation mediator production from microglia via NF-kappa B and MAPK pathways. Chin J Integr Med. 2019; 25:131–138. PMID: 29790065.24. Jeong JW, Lee HH, Choi EO, Lee KW, Kim KY, Kim SG, Hong SH, Kim GY, Park C, Kim HK, Choi YW, Choi YH. Schisandrae Fructus inhibits IL-1beta-induced matrix metalloproteinases and inflammatory mediators production in SW1353 human chondrocytes by suppressing NF-kappaB and MAPK activation. Drug Dev Res. 2015; 76:474–483. PMID: 26443270.25. Dilshara MG, Jayasooriya RG, Kang CH, Lee S, Park SR, Jeong JW, Choi YH, Seo YT, Jang YP, Kim GY. Downregulation of pro-inflammatory mediators by a water extract of Schisandra chinensis (Turcz.) Baill fruit in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Environ Toxicol Pharmacol. 2013; 36:256–264. PMID: 23686005.26. Kang JS, Han MH, Kim GY, Kim CM, Kim BW, Hwang HJ, Hyun Y. Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients. 2014; 6:5667–5678. PMID: 25493944.27. Karna KK, Choi BR, Kim MJ, Kim HK, Park JK. The effect of Schisandra chinensis Baillon on cross-talk between oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in testes of varicocele-induced SD rat. Int J Mol Sci. 2019; 20:5785.28. Jeong JW, Kim J, Choi EO, Kwon DH, Kong GM, Choi IW, Kim BH, Kim GY, Lee KW, Kim KY, Kim SG, Choi YW, Hong SH, Park C, Choi YH. Schisandrae Fructus ethanol extract ameliorates inflammatory responses and articular cartilage damage in monosodium iodoacetate-induced osteoarthritis in rats. EXCLI J. 2017; 16:265–277. PMID: 28507472.29. Choi YH. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Genomics. 2021; 43:303–312. PMID: 32851512.
Article30. Chae BS. Effect of low-dose corticosterone pretreatment on the production of inflammatory mediators in super-low-dose LPS-primed immune cells. Toxicol Res. 2021; 37:47–57. PMID: 33489857.
Article31. Park S, Kim M, Hong Y, Lee H, Tran Q, Kim C, Kwon SH, Park J, Park J, Kim SH. Myristoylated TMEM39AS41, a cell-permeable peptide, causes lung cancer cell death. Toxicol Res. 2020; 36:123–130. PMID: 32257924.
Article32. Park JW, Lee SJ, Kim JE, Kang MJ, Bae SJ, Choi YJ, Gong JE, Kim KS, Jung YS, Cho JY, Choi YS, Hwang DY, Song HK. Comparison of response to LPS-induced sepsis in three DBA/2 stocks derived from different sources. Lab Anim Res. 2021; 37:2. PMID: 33407886.
Article33. Hwangbo H, Kim SY, Lee H, Park SH, Hong SH, Park C, Kim GY, Leem SH, Hyun JW, Cheong J, Choi YH. Auranofin enhances sulforaphane-mediated apoptosis in hepatocellular carcinoma Hep3B cells through inactivation of the PI3K/Akt signaling pathway. Biomol Ther. 2020; 28:443–455.
Article34. Jeong JW, Cha HJ, Han MH, Hwang SJ, Lee DS, Yoo JS, Choi IW, Kim S, Kim HS, Kim GY, Hong SH, Park C, Lee HJ, Choi YH. Spermidine protects against oxidative stress in inflammation models using macrophages and zebrafish. Biomol Ther. 2018; 26:146–156.
Article35. Soufli I, Toumi R, Rafa H, Touil-Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016; 7:353–360. PMID: 27602236.
Article36. Aleem D, Tohid H. Pro-inflammatory cytokines, biomarkers, genetics and the immune system: a mechanistic approach of depression and psoriasis. Rev Colomb Psiquiatr. 2018; 47:177–186.
Article37. Saini R, Singh S. Inducible nitric oxide synthase: an asset to neutrophils. J Leukoc Biol. 2019; 105:49–61. PMID: 30285282.
Article38. Yao C, Narumiya S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br J Pharmacol. 2019; 176:337–354. PMID: 30381825.
Article39. Wang T, He C, Yu X. Pro-inflammatory cytokines: new potential therapeutic targets for obesity-related bone disorders. Curr Drug Targets. 2017; 18:1664–1675. PMID: 28056748.
Article40. Hu F, Lou N, Jiao J, Guo F, Xiang H, Shang D. Macrophages in pancreatitis: Mechanisms and therapeutic potential. Biomed Pharmacother. 2020; 131:110693. PMID: 32882586.
Article41. Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020; 28:555–561. PMID: 31982565.
Article42. Jalava PI, Salonen RO, Pennanen AS, Happo MS, Penttinen P, Hälinen AI, Sillanpää M, Hillamo R, Hirvonen MR. Effects of solubility of urban air fine and coarse particles on cytotoxic and inflammatory responses in RAW 264.7 macrophage cell line. Toxicol Appl Pharmacol. 2008; 229:146–160. PMID: 18325559.
Article43. Bekki K, Ito T, Yoshida Y, He C, Arashidani K, He M, Sun G, Zeng Y, Sone H, Kunugita N, Ichinose T. PM2.5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways. Environ Toxicol Pharmacol. 2016; 45:362–369. PMID: 27393915.44. Zhu J, Zhao Y, Gao Y, Li C, Zhou L, Qi W, Zhang Y, Ye L. Effects of different components of PM2.5 on the expression levels of NF-kappaB family gene mRNA and inflammatory molecules in human macrophage. Int J Environ Res Public Health. 2019; 16:1408.45. Yin J, Xia W, Li Y, Guo C, Zhang Y, Huang S, Jia Z, Zhang A. COX-2 mediates PM2.5-induced apoptosis and inflammation in vascular endothelial cells. Am J Transl Res. 2017; 9:3967–3976. PMID: 28979673.46. Beck-Speier I, Kreyling WG, Maier KL, Dayal N, Schladweiler MC, Mayer P, Semmler-Behnke M, Kodavanti UP. Soluble iron modulates iron oxide particle-induced inflammatory responses via prostaglandin E2 synthesis: in vitro and in vivo studies. Part Fibre Toxicol. 2009; 6:34. PMID: 20028532.
Article47. Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. Adverse effects of wood smoke PM2.5 exposure on macrophage functions. Inhal Toxicol. 2013; 25:67–76. PMID: 23363038.48. Li W, Cai ZN, Mehmood S, Liang LL, Liu Y, Zhang HY, Chen Y, Lu YM. Anti-inflammatory effects of Morchella esculenta polysaccharide and its derivatives in fine particulate matter-treated NR8383 cells. Int J Biol Macromol. 2019; 129:904–915. PMID: 30776439.49. Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013; 45:2580–2584. PMID: 24004831.
Article50. Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015; 5:1266–1283. PMID: 26131974.
Article51. Shi Y, Batibawa JW, Maiga M, Sun B, Li Y, Duan J, Sun Z. Identification and validation of metformin protects against PM2.5-induced macrophages cytotoxicity by targeting toll like receptor pathway. Chemosphere. 2020; 251:126526. PMID: 32443237.52. Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front Physiol. 2018; 9:477. PMID: 29867535.
Article53. Mills EL, O'Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol. 2016; 46:13–21. PMID: 26643360.
Article54. Bailone RL, Fukushima HC, Ventura Fernandes BH, De Aguiar LK, Corrêa T, Janke H, Grejo Setti P, Roça RO, Borra RC. Zebrafish as an alternative animal model in human and animal vaccination research. Lab Anim Res. 2020; 36:13. PMID: 32382525.
Article55. Ren F, Huang Y, Tao Y, Ji C, Aniagu S, Jiang Y, Chen T. Resveratrol protects against PM2.5-induced heart defects in zebrafish embryos as an antioxidant rather than as an AHR antagonist. Toxicol Appl Pharmacol. 2020; 398:115029. PMID: 32376357.
Article56. Zhang Y, Li S, Li J, Han L, He Q, Wang R, Wang X, Liu K. Developmental toxicity induced by PM2.5 through endoplasmic reticulum stress and autophagy pathway in zebrafish embryos. Chemosphere. 2018; 197:611–621. PMID: 29407824.
Article57. Dai YL, Jiang YF, Lu YA, Yu JB, Kang MC, Jeon YJ. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo . Toxicol Rep. 2021; 8:349–358. PMID: 33665132.58. de Abreu MS, Giacomini AC, Genario R, Dos Santos BE, da Rosa LG, Demin KA, Wappler-Guzzetta EA, Kalueff AV. Neuropharmacology, pharmacogenetics and pharmacogenomics of aggression: the zebrafish model. Pharmacol Res. 2019; 141:602–608. PMID: 30708051.
Article59. Duan J, Yu Y, Li Y, Wang Y, Sun Z. Inflammatory response and blood hypercoagulable state induced by low level co-exposure with silica nanoparticles and benzo[a]pyrene in zebrafish (Danio rerio) embryos. Chemosphere. 2016; 151:152–162. PMID: 26943738.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Negative Air Ions Alleviate Particulate Matter-Induced Inflammation and Oxidative Stress in the Human Keratinocyte Cell Line HaCaT
- Protective effect of Evodiae Fructus extract in HCl/ethanol-induced gastritis mice
- Role of Particulate Matter in Skin Inflammation
- Anti-Toxoplasmosis Effect of Meliae fructus Ethanol Extract
- Role of NO in Activation of NFkB by PM 2.5 in Lung Epithelial Cells